Cyanobacteria as a Platform for Biofuel Production
- PMID: 25022311
- PMCID: PMC4090892
- DOI: 10.3389/fbioe.2013.00007
Cyanobacteria as a Platform for Biofuel Production
Abstract
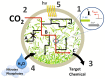
Cyanobacteria have great potential as a platform for biofuel production because of their fast growth, ability to fix carbon dioxide gas, and their genetic tractability. Furthermore they do not require fermentable sugars or arable land for growth and so competition with cropland would be greatly reduced. In this perspective we discuss the challenges and areas for improvement most pertinent for advancing cyanobacterial fuel production, including: improving genetic parts, carbon fixation, metabolic flux, nutrient requirements on a large scale, and photosynthetic efficiency using natural light.
Keywords: biofuel; cyanobacteria; metabolic engineering; photosynthesis; synthetic biology.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

