Photoclick chemistry: a fluorogenic light-triggered in vivo ligation reaction
- PMID: 25022432
- PMCID: PMC4149939
- DOI: 10.1016/j.cbpa.2014.05.024
Photoclick chemistry: a fluorogenic light-triggered in vivo ligation reaction
Abstract
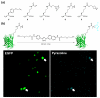
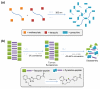
The ability to use chemical reactivity to monitor and control biomolecular processes with a spatial and temporal precision motivated the development of light-triggered in vivo chemistries. To this end, the photoinduced tetrazole-alkene cycloaddition, also termed 'photoclick chemistry' offers a very rapid chemical ligation platform for the manipulation of biomolecules and matrices in vivo. Here we outline the recent developments in the optimization of this chemistry, ranging from the search for substrates that offer two-photon photoactivatability, superior reaction kinetics, and/or genetic encodability, to the study of the reaction mechanism. The applications of the photoclick chemistry in protein labeling in vitro and in vivo as well as in preparing 'smart' hydrogels for 3D cell culture are highlighted.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures

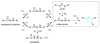
References
-
- Tasdelen MA, Yagci Y. Light-induced click reactions. Angew Chem Intl Ed. 2013;52:5930–5938. - PubMed
-
- Clovis JS, Eckell A, Huisgen R, Sustmann R. 1.3-dipolare cycloadditionen, xxv. Der nachweis des freien diphenylnitrilimins als zwischenstufe bei cycloadditionen. Chem Ber. 1967;100:60–70.
-
-
Song W, Wang Y, Qu J, Madden MM, Lin Q. A photoinducible 1,3-dipolar cycloaddition reaction for rapid, selective modification of tetrazole-containing proteins. Angew Chem Intl Ed. 2008;47:2832–2835. This was the first report of the tetrazole-alkene photoclick chemistry as a new bioorthogonal reaction for biological applications.
-
-
-
Song W, Wang Y, Qu J, Lin Q. Selective functionalization of a genetically encoded alkene-containing protein via “photoclick chemistry” in bacterial cells. J Am Chem Soc. 2008;130:9654–9655. This report established the suitability of photoclick chemistry for in vivo protein labeling.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

