The influence of cerebrospinal fluid (CSF) biomarkers on clinical dementia evaluations
- PMID: 25022536
- PMCID: PMC4287458
- DOI: 10.1016/j.jalz.2014.04.517
The influence of cerebrospinal fluid (CSF) biomarkers on clinical dementia evaluations
Abstract
Background: Cerebrospinal fluid (CSF) proteins have become accepted biomarkers of Alzheimer's disease (AD) in research settings. The extent of their use, perceived utility, and influence on decision making in clinical settings, however, are less well studied.
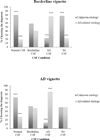
Methods: Clinicians who evaluate older adults (N = 193) were randomized to view normal, borderline, AD-consistent, or no CSF information in two vignettes portraying patients with borderline and mild AD symptoms. Clinicians also reported on the use and perceived utility of CSF biomarkers.
Results: Although clinicians reported infrequent use and low perceived utility of CSF biomarkers, viewing AD-consistent CSF values made clinicians more likely to make an AD-related diagnosis, increased diagnostic confidence, and led clinicians to initiate treatment more often than clinicians who had no CSF information.
Conclusions: CSF biomarkers influence decision making depending on the extent to which biomarkers reflect AD pathology, consistency between clinical-pathologic information, and the ambiguity of protein values.
Keywords: Alzheimer's disease; Biomarkers; Cerebrospinal fluid; Dementia; Diagnosis.
Copyright © 2015 The Alzheimer's Association. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures
References
-
- Dubois B, Feldman HH, Jacova C, Cummings JL, DeKosky S, Barberger-Gateau P, et al. Revising the definition of Alzheimer’s disease: A new lexicon. Lancet Neurol. 2010;9(11):1118–1127. - PubMed
-
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, et al. The diagnosis of dementia due to Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–269. - PMC - PubMed
-
- Blennow K, Zetterberg H. Cerebrospinal fluid biomarkers for Alzheimer’s disease. J Alzheimers Dis. 2009;18(2):413–417. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical