Natural killer cells and regulatory T cells in early pregnancy loss
- PMID: 25023688
- PMCID: PMC4306453
- DOI: 10.1387/ijdb.140109ss
Natural killer cells and regulatory T cells in early pregnancy loss
Abstract
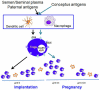
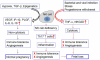
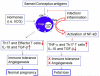
Survival of the allogeneic embryo in the uterus depends on the maintenance of immune tolerance at the maternal-fetal interface. The pregnant uterus is replete with activated maternal immune cells. How this immune tolerance is acquired and maintained has been a topic of intense investigation. The key immune cells that predominantly populate the pregnant uterus are natural killer (NK) cells. In normal pregnancy, these cells are not killers, but rather provide a microenvironment that is pregnancy compatible and supports healthy placentation. In placental mammals, an array of highly orchestrated immune elements to support successful pregnancy outcome has been incorporated. This includes active cooperation between maternal immune cells, particularly NK cells, and trophoblast cells. This intricate process is required for placentation, immune regulation and to remodel the blood supply to the fetus. During the past decade, various types of maternal immune cells have been thought to be involved in cross-talk with trophoblasts and in programming immune tolerance. Regulatory T cells (Tregs) have attracted a great deal of attention in promoting implantation and immune tolerance beyond implantation. However, what has not been fully addressed is how this immune-trophoblast axis breaks down during adverse pregnancy outcomes, particularly early pregnancy loss, and in response to unscheduled inflammation. Intense research efforts have begun to shed light on the roles of NK cells and Tregs in early pregnancy loss, although much remains to be unraveled in order to fully characterize the mechanisms underlying their detrimental activity. An increased understanding of host-environment interactions that lead to the cytotoxic phenotype of these otherwise pregnancy compatible maternal immune cells is important for prediction, prevention and treatment of pregnancy maladies, particularly recurrent pregnancy loss. In this review, we discuss relevant information from experimental and human models that may explain the pregnancy disrupting roles of these pivotal sentinel cells at the maternal-fetal interface.
Figures
References
-
- ALUVIHARE VR, KALLIKOURDIS M, BETZ AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol. 2004;5:266–271. - PubMed
-
- ARNON TI, MARKEL G, MANDELBOIM O. Tumor and viral recognition by natural killer cells receptors. Sem Cancer Biol. 2006;16:348–358. - PubMed
-
- ASHKAR AA, CROY BA. Interferon-gamma contributes to the normalcy of murine pregnancy. Biol Reprod. 1999;61:493–502. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

