Cholesterol selectively activates canonical Wnt signalling over non-canonical Wnt signalling
- PMID: 25024088
- PMCID: PMC4100210
- DOI: 10.1038/ncomms5393
Cholesterol selectively activates canonical Wnt signalling over non-canonical Wnt signalling
Abstract
Wnt proteins control diverse biological processes through β-catenin-dependent canonical signalling and β-catenin-independent non-canonical signalling. The mechanisms by which these signalling pathways are differentially triggered and controlled are not fully understood. Dishevelled (Dvl) is a scaffold protein that serves as the branch point of these pathways. Here, we show that cholesterol selectively activates canonical Wnt signalling over non-canonical signalling under physiological conditions by specifically facilitating the membrane recruitment of the PDZ domain of Dvl and its interaction with other proteins. Single-molecule imaging analysis shows that cholesterol is enriched around the Wnt-activated Frizzled and low-density lipoprotein receptor-related protein 5/6 receptors and plays an essential role for Dvl-mediated formation and maintenance of the canonical Wnt signalling complex. Collectively, our results suggest a new regulatory role of cholesterol in Wnt signalling and a potential link between cellular cholesterol levels and the balance between canonical and non-canonical Wnt signalling activities.
Figures





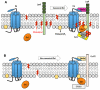
References
-
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 2004;20:781–810. - PubMed
-
- Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. - PubMed
-
- Klaus A, Birchmeier W. Wnt signalling and its impact on development and cancer. Nat. Rev. Cancer. 2008;8:387–398. - PubMed
-
- Gao C, Chen YG. Dishevelled: The hub of Wnt signaling. Cell. Signal. 2010;22:717–727. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

