Platelets guide the formation of early metastatic niches
- PMID: 25024172
- PMCID: PMC4121772
- DOI: 10.1073/pnas.1411082111
Platelets guide the formation of early metastatic niches
Abstract
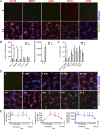
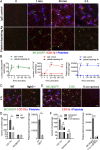
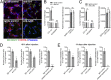
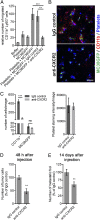
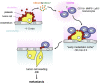
During metastasis, host cells are recruited to disseminated tumor cells to form specialized microenvironments ("niches") that promote metastatic progression, but the mechanisms guiding the assembly of these niches are largely unknown. Tumor cells may autonomously recruit host cells or, alternatively, host cell-to-host cell interactions may guide the formation of these prometastatic microenvironments. Here, we show that platelet-derived rather than tumor cell-derived signals are required for the rapid recruitment of granulocytes to tumor cells to form "early metastatic niches." Granulocyte recruitment relies on the secretion of CXCL5 and CXCL7 chemokines by platelets upon contact with tumor cells. Blockade of the CXCL5/7 receptor CXCR2, or transient depletion of either platelets or granulocytes prevents the formation of early metastatic niches and significantly reduces metastatic seeding and progression. Thus, platelets recruit granulocytes and guide the formation of early metastatic niches, which are crucial for metastasis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Wolf MJ, et al. Endothelial CCR2 signaling induced by colon carcinoma cells enables extravasation via the JAK2-Stat5 and p38MAPK pathway. Cancer Cell. 2012;22(1):91–105. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

