Caspase-8 promotes NLRP1/NLRP3 inflammasome activation and IL-1β production in acute glaucoma
- PMID: 25024200
- PMCID: PMC4121847
- DOI: 10.1073/pnas.1402819111
Caspase-8 promotes NLRP1/NLRP3 inflammasome activation and IL-1β production in acute glaucoma
Abstract
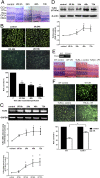
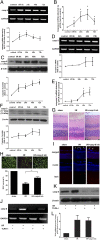
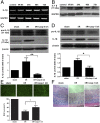
Acute glaucoma is a sight-threatening condition characterized by a sudden and substantial rise in intraocular pressure (IOP) and consequent retinal ganglion cell (RGC) death. Angle closure glaucoma, a common cause of glaucoma in Asia that affects tens of millions of people worldwide, often presents acutely with loss of vision, pain, and high IOP. Even when medical and surgical treatment is available, acute angle closure glaucoma can cause permanent and irreversible loss of vision. Toll-like receptor 4 (TLR4) signaling has been previously implicated in the pathogenesis of IOP-induced RGC death, although the underlying mechanisms are largely unknown. In the present study, we used an acute IOP elevation/glaucoma model to investigate the underlying mechanism of RGC death. We found that TLR4 leads to increased caspase-8 expression; this elevation increases IL-1β expression and RGC death via a caspase-1-dependent pathway involving Nod-like receptor family, pyrin domain containing 1 (NLRP1)/NLRP3 inflammasomes and a caspase-1-independent pathway. We show that inhibition of caspase-8 activation significantly attenuates RGC death by down-regulating the activation of NLRP1 and NLRP3, thus demonstrating the pivotal role of caspase-8 in the TLR4-mediated activation of inflammasomes. These findings demonstrate collectively a critical role of caspase-8 in transducing TLR4-mediated IL-1β production and RGC death and highlight signal transduction in a caspase-1-dependent NLRP1/NLRP3 inflammasome pathway and a caspase-1-independent pathway in acute glaucoma. These results provide new insight into the pathogenesis of glaucoma and point to a treatment strategy.
Keywords: cell apoptosis; retinal ischemia/reperfusion injury.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Wang N, Wu H, Fan Z. Primary angle closure glaucoma in Chinese and Western populations. Chin Med J (Engl) 2002;115(11):1706–1715. - PubMed
-
- Ang LP, Ang LP. Current understanding of the treatment and outcome of acute primary angle-closure glaucoma: An Asian perspective. Ann Acad Med Singapore. 2008;37(3):210–215. - PubMed
-
- Quek DT, et al. Blindness and long-term progression of visual field defects in chinese patients with primary angle-closure glaucoma. Am J Ophthalmol. 2011;152(3):463–469. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

