prickle modulates microtubule polarity and axonal transport to ameliorate seizures in flies
- PMID: 25024231
- PMCID: PMC4121842
- DOI: 10.1073/pnas.1403357111
prickle modulates microtubule polarity and axonal transport to ameliorate seizures in flies
Abstract
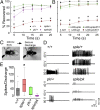
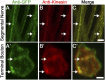
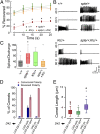
Recent analyses in flies, mice, zebrafish, and humans showed that mutations in prickle orthologs result in epileptic phenotypes, although the mechanism responsible for generating the seizures was unknown. Here, we show that Prickle organizes microtubule polarity and affects their growth dynamics in axons of Drosophila neurons, which in turn influences both anterograde and retrograde vesicle transport. We also show that enhancement of the anterograde transport mechanism is the cause of the seizure phenotype in flies, which can be suppressed by reducing the level of either of two Kinesin motor proteins responsible for anterograde vesicle transport. Additionally, we show that seizure-prone prickle mutant flies have electrophysiological defects similar to other fly mutants used to study seizures, and that merely altering the balance of the two adult prickle isoforms in neurons can predispose flies to seizures. These data reveal a previously unidentified pathway in the pathophysiology of seizure disorders and provide evidence for a more generalized cellular mechanism whereby Prickle mediates polarity by influencing microtubule-mediated transport.
Keywords: EB1-GFP; epilepsy; neurodegeneration; planar cell polarity; spiny-legs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

