Electronic health records for intervention research: a cluster randomized trial to reduce antibiotic prescribing in primary care (eCRT study)
- PMID: 25024243
- PMCID: PMC4096472
- DOI: 10.1370/afm.1659
Electronic health records for intervention research: a cluster randomized trial to reduce antibiotic prescribing in primary care (eCRT study)
Abstract
Purpose: This study aimed to implement a point-of-care cluster randomized trial using electronic health records. We evaluated the effectiveness of electronically delivered decision support tools at reducing antibiotic prescribing for respiratory tract infections in primary care.
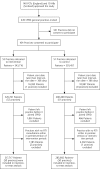
Methods: Family practices from England and Scotland participating in the Clinical Practice Research Datalink (CPRD) were included in the trial; 53 family practices were allocated to intervention and 51 practices were allocated to usual care. Patients aged 18 to 59 years consulting for respiratory tract infections were eligible. The intervention was through remotely installed, computer-delivered decision support tools accessed during the consultations. Control practices provided usual care. The primary outcome was the proportion of consultations for respiratory tract infections with an antibiotic prescribed based on electronic health records. Family practice-specific proportions were included in a cluster-level analysis.
Results: Data were analyzed for 603,409 patients: 317,717 at intervention practices and 285,692 at control practices. Use of the intervention was less than anticipated, varying among practices. There was a reduction in proportion of consultations with antibiotics prescribed of 1.85% (95% CI, 0.10%-3.59%, P=.038) and in the rate of antibiotic prescribing for respiratory tract infections (9.69%; 95% CI, 0.75%-18.63%, fewer prescriptions per 1,000 patient-years, P=.034). There were no adverse events.
Conclusions: Cluster randomized trials may be implemented efficiently in large samples from routine care settings by using primary care electronic health records. Future studies should develop and test multicomponent methods for remotely delivered intervention.
Keywords: antibiotic; electronic health records; primary health care; randomized controlled trial; respiratory tract infection.
© 2014 Annals of Family Medicine, Inc.
Figures
References
-
- Schwartz D, Lellouch J. Explanatory and pragmatic attitudes in therapeutical trials. J Clin Epidemiol. 2009;62(5):499–505 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
