Disrupted coupling of gating charge displacement to Na+ current activation for DIIS4 mutations in hypokalemic periodic paralysis
- PMID: 25024265
- PMCID: PMC4113897
- DOI: 10.1085/jgp.201411199
Disrupted coupling of gating charge displacement to Na+ current activation for DIIS4 mutations in hypokalemic periodic paralysis
Abstract
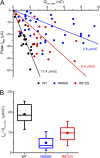
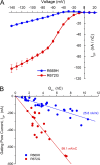
Missense mutations at arginine residues in the S4 voltage-sensor domains of NaV1.4 are an established cause of hypokalemic periodic paralysis, an inherited disorder of skeletal muscle involving recurrent episodes of weakness in conjunction with low serum K(+). Expression studies in oocytes have revealed anomalous, hyperpolarization-activated gating pore currents in mutant channels. This aberrant gating pore conductance creates a small inward current at the resting potential that is thought to contribute to susceptibility to depolarization in low K(+) during attacks of weakness. A critical component of this hypothesis is the magnitude of the gating pore conductance relative to other conductances that are active at the resting potential in mammalian muscle: large enough to favor episodes of paradoxical depolarization in low K(+), yet not so large as to permanently depolarize the fiber. To improve the estimate of the specific conductance for the gating pore in affected muscle, we sequentially measured Na(+) current through the channel pore, gating pore current, and gating charge displacement in oocytes expressing R669H, R672G, or wild-type NaV1.4 channels. The relative conductance of the gating pore to that of the pore domain pathway for Na(+) was 0.03%, which implies a specific conductance in muscle from heterozygous patients of ∼ 10 µS/cm(2) or 1% of the total resting conductance. Unexpectedly, our data also revealed a substantial decoupling between gating charge displacement and peak Na(+) current for both R669H and R672G mutant channels. This decoupling predicts a reduced Na(+) current density in affected muscle, consistent with the observations that the maximal dV/dt and peak amplitude of the action potential are reduced in fibers from patients with R672G and in a knock-in mouse model of R669H. The defective coupling between gating charge displacement and channel activation identifies a previously unappreciated mechanism that contributes to the reduced excitability of affected fibers seen with these mutations and possibly with other R/X mutations of S4 of NaV, CaV, and KV channels associated with human disease.
© 2014 Mi et al.
Figures


Similar articles
-
A Na+ channel mutation linked to hypokalemic periodic paralysis exposes a proton-selective gating pore.J Gen Physiol. 2007 Jul;130(1):11-20. doi: 10.1085/jgp.200709755. J Gen Physiol. 2007. PMID: 17591984 Free PMC article.
-
Gating pore currents in DIIS4 mutations of NaV1.4 associated with periodic paralysis: saturation of ion flux and implications for disease pathogenesis.J Gen Physiol. 2008 Oct;132(4):447-64. doi: 10.1085/jgp.200809967. J Gen Physiol. 2008. PMID: 18824591 Free PMC article.
-
A sodium channel knockin mutant (NaV1.4-R669H) mouse model of hypokalemic periodic paralysis.J Clin Invest. 2011 Oct;121(10):4082-94. doi: 10.1172/JCI57398. Epub 2011 Sep 1. J Clin Invest. 2011. PMID: 21881211 Free PMC article.
-
When muscle Ca2+ channels carry monovalent cations through gating pores: insights into the pathophysiology of type 1 hypokalaemic periodic paralysis.J Physiol. 2018 Jun;596(11):2019-2027. doi: 10.1113/JP274955. Epub 2018 Apr 15. J Physiol. 2018. PMID: 29572832 Free PMC article. Review.
-
Gating Pore Currents in Sodium Channels.Handb Exp Pharmacol. 2018;246:371-399. doi: 10.1007/164_2017_54. Handb Exp Pharmacol. 2018. PMID: 28965172 Review.
Cited by
-
Mice with an NaV1.4 sodium channel null allele have latent myasthenia, without susceptibility to periodic paralysis.Brain. 2016 Jun;139(Pt 6):1688-99. doi: 10.1093/brain/aww070. Epub 2016 Apr 5. Brain. 2016. PMID: 27048647 Free PMC article.
-
New Challenges Resulting From the Loss of Function of Nav1.4 in Neuromuscular Diseases.Front Pharmacol. 2021 Oct 4;12:751095. doi: 10.3389/fphar.2021.751095. eCollection 2021. Front Pharmacol. 2021. PMID: 34671263 Free PMC article. Review.
-
Voltage-dependent Ca2+ release is impaired in hypokalemic periodic paralysis caused by CaV1.1-R528H but not by NaV1.4-R669H.Am J Physiol Cell Physiol. 2022 Aug 1;323(2):C478-C485. doi: 10.1152/ajpcell.00209.2022. Epub 2022 Jun 27. Am J Physiol Cell Physiol. 2022. PMID: 35759432 Free PMC article.
-
Gating pore currents occur in CaV1.1 domain III mutants associated with HypoPP.J Gen Physiol. 2021 Nov 1;153(11):e202112946. doi: 10.1085/jgp.202112946. Epub 2021 Aug 31. J Gen Physiol. 2021. PMID: 34463712 Free PMC article.
-
Phospholemman, a major regulator of skeletal muscle Na+/K+-ATPase, is not mutated in probands with hypokalemic periodic paralysis.Exp Ther Med. 2017 Oct;14(4):3229-3232. doi: 10.3892/etm.2017.4848. Epub 2017 Jul 28. Exp Ther Med. 2017. PMID: 28912873 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

