Targeting cancer stem cell plasticity through modulation of epidermal growth factor and insulin-like growth factor receptor signaling in head and neck squamous cell cancer
- PMID: 25024430
- PMCID: PMC4149297
- DOI: 10.5966/sctm.2013-0214
Targeting cancer stem cell plasticity through modulation of epidermal growth factor and insulin-like growth factor receptor signaling in head and neck squamous cell cancer
Abstract
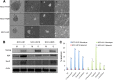
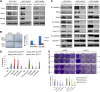
Emerging data suggest that cancer stem cells (CSCs) exist in equilibrium with differentiated cells and that stochastic transitions between these states can account for tumor heterogeneity and drug resistance. The aim of this study was to establish an in vitro system that recapitulates stem cell plasticity in head and neck squamous cell cancers (HNSCCs) and identify the factors that play a role in the maintenance and repopulation of CSCs. Tumor spheres were established using patient-derived cell lines via anchorage-independent cell culture techniques. These tumor spheres were found to have higher aldehyde dehydrogenase (ALD) cell fractions and increased expression of Kruppel-like factor 4, SRY (sex determining region Y)-box 2, and Nanog and were resistant to γ-radiation, 5-fluorouracil, cisplatin, and etoposide treatment compared with monolayer culture cells. Monolayer cultures were subject to single cell cloning to generate clones with high and low ALD fractions. ALDHigh clones showed higher expression of stem cell and epithelial-mesenchymal transition markers compared with ALDLow clones. ALD fractions, representing stem cell fractions, fluctuated with serial passaging, equilibrating at a level specific to each cell line, and could be augmented by the addition of epidermal growth factor (EGF) and/or insulin. ALDHigh clones showed increased EGF receptor (EGFR) and insulin-like growth factor-1 receptor (IGF-1R) phosphorylation, with increased activation of downstream pathways compared with ALDLow clones. Importantly, blocking these pathways using specific inhibitors against EGFR and IGF-1R reduced stem cell fractions drastically. Taken together, these results show that HNSCC CSCs exhibit plasticity, with the maintenance of the stem cell fraction dependent on the EGFR and IGF-1R pathways and potentially amenable to targeted therapeutics.
Keywords: Aldehyde dehydrogenase; Cancer stem cell; Epidermal growth factor receptor; Head and neck squamous cell cancer; Insulin-like growth factor-1 receptor.
©AlphaMed Press.
Figures



References
-
- Hammerman PS, Jänne PA, Johnson BE. Resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer. Clin Cancer Res. 2009;15:7502–7509. - PubMed
-
- Ferlay J, Shin H, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. - PubMed
-
- Vermorken JB, Specenier P. Optimal treatment for recurrent/metastatic head and neck cancer. Ann Oncol. 2010;21(suppl 7):vii252–vii261. - PubMed
-
- Medema JP. Cancer stem cells: the challenges ahead. Nat Cell Biol. 2013;15:338–344. - PubMed
-
- Wicha MS, Liu S, Dontu G. Cancer stem cells: An old idea—A paradigm shift. Cancer Res. 2006;66:1883–1890. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

