The impact of Staphylococcus aureus-associated molecular patterns on staphylococcal superantigen-induced toxic shock syndrome and pneumonia
- PMID: 25024509
- PMCID: PMC4082930
- DOI: 10.1155/2014/468285
The impact of Staphylococcus aureus-associated molecular patterns on staphylococcal superantigen-induced toxic shock syndrome and pneumonia
Abstract
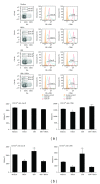
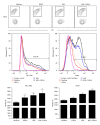
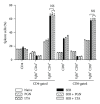
Staphylococcus aureus is capable of causing a spectrum of human illnesses. During serious S. aureus infections, the staphylococcal pathogen-associated molecular patterns (PAMPs) such as peptidoglycan, lipoteichoic acid, and lipoproteins and even intact S. aureus, are believed to act in conjunction with the staphylococcal superantigens (SSAg) to activate the innate and adaptive immune system, respectively, and cause immunopathology. However, recent studies have shown that staphylococcal PAMPs could suppress inflammation by several mechanisms and protect from staphylococcal toxic shock syndrome, a life-threatening systemic disease caused by toxigenic S. aureus. Given the contradictory pro- and anti-inflammatory roles of staphylococcal PAMPs, we examined the effects of S. aureus-derived molecular patterns on immune responses driven by SSAg in vivo using HLA-DR3 and HLA-DQ8 transgenic mice. Our study showed that neither S. aureus-derived peptidoglycans (PGN), lipoteichoic acid (LTA), nor heat-killed Staphylococcus aureus (HKSA) inhibited SSAg-induced T cell proliferation in vitro. They failed to antagonize the immunostimulatory effects of SSAg in vivo as determined by their inability to attenuate systemic cytokine/chemokine response and reduce SSAg-induced T cell expansion. These staphylococcal PAMPs also failed to protect HLA-DR3 as well as HLA-DQ8 transgenic mice from either SSAg-induced toxic shock or pneumonia induced by a SSAg-producing strain of S. aureus.
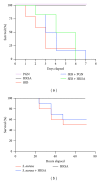
Figures

References
-
- Klevens RM, Morrison MA, Nadle J, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. The Journal of the American Medical Association. 2007;298(15):1763–1771. - PubMed
-
- Boucher HW, Corey GR. Epidemiology of methicillin-resistant Staphylococcus aureus . Clinical Infectious Diseases. 2008;46(supplement 5):S344–S349. - PubMed
-
- Fraser JD, Proft T. The bacterial superantigen and superantigen-like proteins. Immunological Reviews. 2008;225(1):226–243. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

