Evaluation of a Korean version of a tool for assessing the incorporation of human factors into a medication-related decision support system: the I-MeDeSA
- PMID: 25024770
- PMCID: PMC4081757
- DOI: 10.4338/ACI-2014-01-RA-0005
Evaluation of a Korean version of a tool for assessing the incorporation of human factors into a medication-related decision support system: the I-MeDeSA
Abstract
Objective: The Instrument for Evaluating Human-Factor Principles in Medication-Related Decision Support Alerts (I-MeDeSA) was developed recently in the US with a view towards improving considerations of human-factor principles when designing alerts for clinical decision support (CDS) systems. This study evaluated the generalizability of this tool, in cooperation with its authors, across cultures by applying it to a Korean system. We also examined opportunities to promote user acceptance of the system.
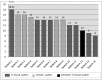
Methods: We developed a Korean version of the I-MeDeSA (K-I-MeDeSA) and used it to evaluate drug-drug interaction alerts in a large academic tertiary hospital in Seoul. We involved four reviewers (A, B, C, and D). Two (A and B) conducted the initial independent scoring, while the other two (C and D) performed a final review and assessed feedback from the initial reviewers. The obtained scores were compared with those from 13 previously reported CDS systems. The feedback was summarized qualitatively.
Results: The translation of the I-MeDeSA had excellent interrater agreement in terms of face validity (scale-level content validity index = 0.95). The system's K-I-MeDeSA score was 10 out of 26, with a good agreement between reviewers (κ = 0.77), which showed a lack of human-factor considerations. The reviewers readily identified two of the nine principles that needed primary improvement: prioritization and text-based information. The reviewers also expressed difficulty judging the following four principles: alarm philosophy, visibility, color, and learnability and confusability.
Conclusion: The K-I-MeDeSA was semantically and operationally equivalent to the original tool. Only minor cultural problems were identified, leading the reviewers to suggest the need for clarification of certain words plus a more detailed description of the tool's rationale and exemplars. Further evaluation is needed to empirically assess whether the implementation of changes in an electronic health record system could improve the adoption of CDS alerts.
Keywords: Clinical decision support; alert fatigue; computerized order entry; human-computer interaction; interfaces and usability.
Conflict of interest statement
None of the authors has any conflicts of interest.
Figures

References
-
- Linder JA, Ma J, Bates DW, Middleton B, Stafford RS.Electronic health record use and the quality of ambulatory care in the United States. Arch Intern Med 2007; 167(13):1400–1405 - PubMed
-
- Bates DW, Gawande AA.Improving safety with information technology. N Engl J Med 2003; 348(25):2526–24534 - PubMed
-
- Fischer MA, Solomon DH, Teich JM, Avorn J.Conversion from intravenous to oral medications: Assessment of a computerized intervention for hospitalized patients. Arch Intern Med 2003; 163(21):2585–259 - PubMed
-
- Wang SJ, Middleton B, Prosser LA, Bardon CG, Spurr CD, Carchidi PJ, Kittler AF, Goldszer RC, Fairchild DG, Sussman AJ, Kuperman GJ, Bates DW.A cost-benefit analysis of electronic medical records in primary care. Am J Med 2003; 114(5):397–403 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

