Autologous platelet-rich plasma: a biological supplement to enhance adipose-derived mesenchymal stem cell expansion
- PMID: 25025830
- PMCID: PMC4346379
- DOI: 10.1089/ten.TEC.2014.0206
Autologous platelet-rich plasma: a biological supplement to enhance adipose-derived mesenchymal stem cell expansion
Abstract
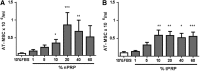
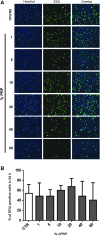
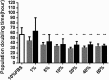
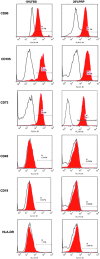
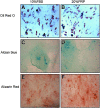
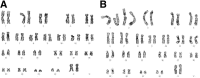
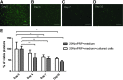
Currently the use of non-autologous cell culture media (e.g., animal-derived or allogeneic serum) for clinical applications of mesenchymal stem cells (MSCs) is criticized by regulatory agencies. Autologous platelet-rich plasma (PRP) is proposed as a safer alternative medium supplement for adipose-derived mesenchymal stem cells (AT-MSC) culture. To study its efficiency on cell proliferation, AT-MSCs were cultured for 10 days in media supplemented with different concentrations of autologous non-activated PRP (nPRP) or thrombin-activated PRP (tPRP) (1-60%). AT-MSC proliferation, cell phenotype, multipotency capacity, and chromosome stability were assessed and compared to AT-MSCs expanded in a classical medium supplemented with 10% of fetal bovine serum (FBS). Culture media supplemented with nPRP showed dose-dependent higher AT-MSC proliferation than did FBS or tPRP. Twenty percent nPRP was the most effective concentration to promote cell proliferation. This condition increased 13.9 times greater AT-MSC number in comparison to culture with FBS, without changing the AT-MSC phenotype, differentiation capacity, and chromosome status. We concluded that 20% autologous nPRP is a safe, efficient, and cost-effective supplement for AT-MSC expansion. It should be considered as an alternative to FBS or other nonautologous blood derivatives. It could serve as a potent substitute for the validation of future clinical protocols as it respects good manufacturing practices and regulatory agencies' standards.
Figures


References
-
- Zhou Y., Yan Z., Zhang H., Lu W., Liu S., Huang X., et al. . Expansion and delivery of adipose-derived mesenchymal stem cells on three microcarriers for soft tissue regeneration. Tissue Eng Part A 17,2981, 2011 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

