Synthesis, antimitotic and antivascular activity of 1-(3',4',5'-trimethoxybenzoyl)-3-arylamino-5-amino-1,2,4-triazoles
- PMID: 25025853
- PMCID: PMC4159078
- DOI: 10.1021/jm5008193
Synthesis, antimitotic and antivascular activity of 1-(3',4',5'-trimethoxybenzoyl)-3-arylamino-5-amino-1,2,4-triazoles
Abstract
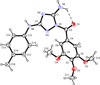
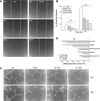
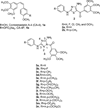
A new class of compounds that incorporated the structural motif of the 1-(3',4',5'-trimethoxtbenzoyl)-3-arylamino-5-amino-1,2,4-triazole molecular skeleton was synthesized and evaluated for their antiproliferative activity in vitro, interactions with tubulin, and cell cycle effects. The most active agent, 3c, was evaluated for antitumor activity in vivo. Structure-activity relationships were elucidated with various substituents on the phenyl ring of the anilino moiety at the C-3 position of the 1,2,4-triazole ring. The best results for inhibition of cancer cell growth were obtained with the p-Me, m,p-diMe, and p-Et phenyl derivatives 3c, 3e, and 3f, respectively, and overall, these compounds were more or less as active as CA-4. Their vascular disrupting activity was evaluated in HUVEC cells, with compound 3c showing activity comparable with that of CA-4. Compound 3c almost eliminated the growth of syngeneic hepatocellular carcinoma in Balb/c mice, suggesting that 3c could be a new antimitotic agent with clinical potential.
Figures







Similar articles
-
Synthesis and biological evaluation of diarylthiazole derivatives as antimitotic and antivascular agents with potent antitumor activity.Bioorg Med Chem. 2015 Jul 1;23(13):3337-50. doi: 10.1016/j.bmc.2015.04.055. Epub 2015 Apr 24. Bioorg Med Chem. 2015. PMID: 25937236
-
A facile synthesis of diaryl pyrroles led to the discovery of potent colchicine site antimitotic agents.Eur J Med Chem. 2021 Mar 15;214:113229. doi: 10.1016/j.ejmech.2021.113229. Epub 2021 Jan 29. Eur J Med Chem. 2021. PMID: 33550186
-
Synthesis and antitumor activity of 1,5-disubstituted 1,2,4-triazoles as cis-restricted combretastatin analogues.J Med Chem. 2010 May 27;53(10):4248-58. doi: 10.1021/jm100245q. J Med Chem. 2010. PMID: 20420439 Free PMC article.
-
Synthesis and biological evaluation of 2- and 3-aminobenzo[b]thiophene derivatives as antimitotic agents and inhibitors of tubulin polymerization.J Med Chem. 2007 May 3;50(9):2273-7. doi: 10.1021/jm070050f. Epub 2007 Apr 10. J Med Chem. 2007. PMID: 17419607
-
Recent Developments on Phenstatins as Potent Antimitotic Agents.Curr Med Chem. 2018;25(20):2329-2352. doi: 10.2174/0929867324666171106162048. Curr Med Chem. 2018. PMID: 29110592 Review.
Cited by
-
Research progress on antitumor activity of XRP44X and analogues as microtubule targeting agents.Front Chem. 2023 Mar 1;11:1096666. doi: 10.3389/fchem.2023.1096666. eCollection 2023. Front Chem. 2023. PMID: 36936533 Free PMC article. Review.
-
Molecular docking studies, biological evaluation and synthesis of novel 3-mercapto-1,2,4-triazole derivatives.Mol Divers. 2021 Feb;25(1):223-232. doi: 10.1007/s11030-020-10050-0. Epub 2020 Feb 17. Mol Divers. 2021. PMID: 32067134
-
Pyrrolo[2',3':3,4]cyclohepta[1,2-d][1,2]oxazoles, a New Class of Antimitotic Agents Active against Multiple Malignant Cell Types.J Med Chem. 2020 Oct 22;63(20):12023-12042. doi: 10.1021/acs.jmedchem.0c01315. Epub 2020 Oct 11. J Med Chem. 2020. PMID: 32986419 Free PMC article.
-
Synthesis of 3-(5-amino-1H-1,2,4-triazol-3-yl)propanamides and their tautomerism.RSC Adv. 2018 Jun 19;8(40):22351-22360. doi: 10.1039/c8ra04576c. eCollection 2018 Jun 19. RSC Adv. 2018. PMID: 35539716 Free PMC article.
-
Targeting tubulin polymerization by novel 7-aryl-pyrroloquinolinones: Synthesis, biological activity and SARs.Eur J Med Chem. 2018 Jan 1;143:244-258. doi: 10.1016/j.ejmech.2017.11.038. Epub 2017 Nov 20. Eur J Med Chem. 2018. PMID: 29197729 Free PMC article.
References
-
- Chen S-M, Meng L-H, Ding J. New microtubule-inhibiting anticancer agents. Expert Opin. Invest. Drugs. 2010;3:329–343. - PubMed
-
- Pettit GR, Singh SB, Hamel E, Lin CM, Alberts DS, Garcia-Kendall D. Isolation and structure of the strong cell growth and tubulin inhibitor combretastatin A-4. Experientia. 1989;45:209–211. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Research Materials
Miscellaneous

