Structural basis for activity of highly efficient RNA mimics of green fluorescent protein
- PMID: 25026079
- PMCID: PMC4143336
- DOI: 10.1038/nsmb.2865
Structural basis for activity of highly efficient RNA mimics of green fluorescent protein
Abstract
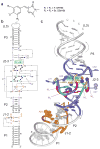
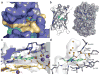
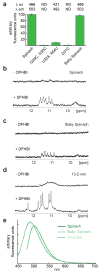
GFP and its derivatives revolutionized the study of proteins. Spinach is a recently reported in vitro-evolved RNA mimic of GFP, which as genetically encoded fusions makes possible live-cell, real-time imaging of biological RNAs without resorting to large RNA-binding protein-GFP fusions. To elucidate the molecular basis of Spinach fluorescence, we solved the cocrystal structure of Spinach bound to its cognate exogenous chromophore, showing that Spinach activates the small molecule by immobilizing it between a base triple, a G-quadruplex and an unpaired G. Mutational and NMR analyses indicate that the G-quadruplex is essential for Spinach fluorescence, is also present in other fluorogenic RNAs and may represent a general strategy for RNAs to induce fluorescence of chromophores. The structure guided the design of a miniaturized 'Baby Spinach', and it provides a foundation for structure-driven design and tuning of fluorescent RNAs.
Conflict of interest statement
S.R.J. and R.L.S. are authors of a patent application (provisional patent USPTO# 61/874,819) related to RNA-fluorophore complexes described in this paper. The other authors declare no competing financial interests.
Figures

References
-
- Tsien RY. The green fluorescent protein. Annu Rev Biochem. 1998;67:509–544. - PubMed
-
- Day RN, Davidson MW, editors. The fluorescent protein revolution. CRC Press; Boca Raton, FL: 2014.
-
- Chalfie M, Tu Y, Euskirchen G, Ward W, Prasher D. Green fluorescent protein as a marker for gene expression. Science. 1994;263:802–805. - PubMed
-
- Ormö M, et al. Crystal structure of the Aequorea victoria green fluorescent protein. Science. 1996;273:1392–1395. - PubMed
-
- Yang F, Moss LG, Phillips GN. The molecular structure of green fluorescent protein. Nature Biotech. 1996;14:1246–1251. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

