Induction of apurinic endonuclease 1 overexpression by endoplasmic reticulum stress in hepatoma cells
- PMID: 25026174
- PMCID: PMC4139852
- DOI: 10.3390/ijms150712442
Induction of apurinic endonuclease 1 overexpression by endoplasmic reticulum stress in hepatoma cells
Abstract
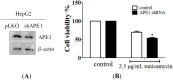
Hepatocellular carcinoma (HCC) is one of the most common malignancies worldwide with poor prognosis due to resistance to conventional chemotherapy and limited efficacy of radiotherapy. Previous studies have noted the induction of endoplasmic reticulum stress or apurinic endonuclease 1 (APE1) expression in many tumors. Therefore, the aim of this study was to investigate the relationship between endoplasmic reticulum (ER stress) and APE1 in hepatocellular carcinoma. Here we investigate the expression of APE1 during ER stress in HepG2 and Huh-7 cell lines. Tunicamycin or brefeldin A, two ER stress inducers, increased APE1 and GRP78, an ER stress marker, expression in HepG2 and Huh-7 cells. Induction of APE1 expression was observed through transcription level in response to ER stress. APE1 nuclear localization during ER stress was determined using immunofluorescence assays in HepG2 cells. Furthermore, expression of Hepatitis B virus pre-S2∆ large mutant surface protein (pre-S2∆), an ER stress-induced protein, also increased GRP78 and APE1 expression in the normal hepatocyte NeHepLxHT cell line. Similarly, tumor samples showed higher expression of APE1 in ER stress-correlated liver cancer tissue in vivo. Our results demonstrate that ER stress and HBV pre-S2∆ increased APE1 expression, which may play an important role in resistance to chemotherapeutic agents or tumor development. Therefore, these data provide an important chemotherapeutic strategy in ER stress and HBV pre-S2∆-associated tumors.
Figures





References
-
- Lee J.W., Soung Y.H., Kim S.Y., Lee H.W., Park W.S., Nam S.W., Kim S.H., Lee J.Y., Yoo N.J., Lee S.H. PIK3CA gene is frequently mutated in breast carcinomas and hepatocellular carcinomas. Oncogene. 2005;24:1477–1480. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

