H-Prune through GSK-3β interaction sustains canonical WNT/β-catenin signaling enhancing cancer progression in NSCLC
- PMID: 25026278
- PMCID: PMC4170634
- DOI: 10.18632/oncotarget.2169
H-Prune through GSK-3β interaction sustains canonical WNT/β-catenin signaling enhancing cancer progression in NSCLC
Abstract
H-Prune hydrolyzes short-chain polyphosphates (PPase activity) together with an hitherto cAMP-phosphodiesterase (PDE), the latest influencing different human cancers by its overexpression. H-Prune promotes cell migration in cooperation with glycogen synthase kinase-3 (Gsk-3β). Gsk-3β is a negative regulator of canonical WNT/β-catenin signaling. Here, we investigate the role of Gsk-3β/h-Prune complex in the regulation of WNT/β-catenin signaling, demonstrating the h-Prune capability to activate WNT signaling also in a paracrine manner, through Wnt3a secretion. In vivo study demonstrates that h-Prune silencing inhibits lung metastasis formation, increasing mouse survival. We assessed h-Prune levels in peripheral blood of lung cancer patients using ELISA assay, showing that h-Prune is an early diagnostic marker for lung cancer. Our study dissects out the mechanism of action of h-Prune in tumorigenic cells and also sheds light on the identification of a new therapeutic target in non-small-cell lung cancer.
Figures
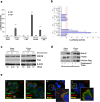
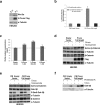


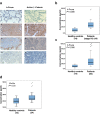
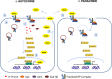
References
-
- D'Angelo A, Garzia L, Andre A, Carotenuto P, Aglio V, Guardiola O, Arrigoni G, Cossu A, Palmieri G, Aravind L, Zollo M. Prune cAMP phosphodiesterase binds nm23-H1 and promotes cancer metastasis. Cancer cell. 2004;5(2):137–149. - PubMed
-
- Diana D, Smaldone G, De Antonellis P, Pirone L, Carotenuto M, Alonzi A, Di Gaetano S, Zollo M, Pedone EM, Fattorusso R. Mapping functional interaction sites of human prune C-terminal domain by NMR spectroscopy in human cell lysates. Chemistry. 2013;19(37):12217–12220. - PubMed
-
- Zollo M, Andre A, Cossu A, Sini MC, D'Angelo A, Marino N, Budroni M, Tanda F, Arrigoni G, Palmieri G. Overexpression of h-prune in breast cancer is correlated with advanced disease status. Clin Cancer Res. 2005;11(1):199–205. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

