Water fleas require microbiota for survival, growth and reproduction
- PMID: 25026374
- PMCID: PMC4274426
- DOI: 10.1038/ismej.2014.116
Water fleas require microbiota for survival, growth and reproduction
Abstract
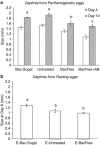
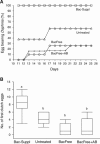
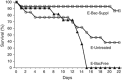
Microbiota have diverse roles in the functioning of their hosts; experiments using model organisms have enabled investigations into these functions. In the model crustacean Daphnia, little knowledge exists about the effect of microbiota on host well being. We assessed the effect of microbiota on Daphnia magna by experimentally depriving animals of their microbiota and comparing their growth, survival and fecundity to that of their bacteria-bearing counterparts. We tested Daphnia coming from both lab-reared parthenogenetic eggs of a single genotype and from genetically diverse field-collected resting eggs. We showed that bacteria-free hosts are smaller, less fecund and have higher mortality than those with microbiota. We also manipulated the association by exposing bacteria-free Daphnia to a single bacterial strain of Aeromonas sp., and to laboratory environmental bacteria. These experiments further demonstrated that the Daphnia-microbiota system is amenable to manipulation under various experimental conditions. The results of this study have implications for studies of D. magna in ecotoxicology, ecology and environmental genomics.
Figures

References
-
- Askarian F, Kousha A, Salma W, Ringo E. The effect of lactic acid bacteria administration on growth, digestive enzyme activity and gut microbiota in Persian sturgeon (Acipenser persicus) and beluga (Huso huso) fry. Aquacult Nutr. 2011;17:488–497.
-
- Bates JM, Mittge E, Kuhlman J, Baden KN, Cheesman SE, Guillemin K. Distinct signals from the microbiota promote different aspects of zebrafish gut differentiation. Dev Biol. 2006;297:374–386. - PubMed
-
- Bleich A, Hansen AK. Time to include the gut microbiota in the hygienic standardisation of laboratory rodents. Comp Immunol Microbiol Infect Dis. 2012;35:81–92. - PubMed
-
- Brucker RM, Bordenstein SR. The hologenomic basis of speciation: gut bacteria cause hybrid lethality in the genus nasonia. Science. 2013;341:667–669. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

