Analysis of three strategies to increase screening coverage for cervical cancer in the general population of women aged 60 to 70 years: the CRICERVA study
- PMID: 25026889
- PMCID: PMC4106208
- DOI: 10.1186/1472-6874-14-86
Analysis of three strategies to increase screening coverage for cervical cancer in the general population of women aged 60 to 70 years: the CRICERVA study
Abstract
Background: Cervical cancer is a frequently diagnosed cancer in women worldwide. Despite having easy preventive and therapeutic approaches, it is an important cause of mortality among women.
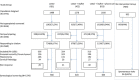
Methods: The CRICERVA study is a cluster clinical trial which assigned one of three interventions to the target population registered in Cerdanyola, Barcelona. Among the 5,707 resident women aged 60 to 70 years in the study area, women with no record of cervical cytology over the last three years were selected. The study included four arms: three interventions all including a pre-assigned date for screening visit and i) personalized invitation letter; ii) adding to i) an informative leaflet; and, iii) in addition to ii) a personalized appointment reminder phone call, and iv) no specific action taken (control group). Participants were offered a personal interview about social-demographic characteristics and about screening attitudes. Cervical cytology and HPV DNA test (HC2) were offered as screening tests. In the case of screening positive in any of these tests, the women were followed up until a full diagnosis could be obtained. The effect size of each study arm was estimated as the absolute gain in coverage between the original coverage and the final coverage.
Results: From the intervention groups (4,775 women), we identified 3,616 who were not appropriately screened, of which 2,560 women answered the trial call and 1,376 were amenable to screening. HPV was tested in 920 women and cervical cytology in all 1,376. Overall, there was an absolute gain in coverage of 28.8% in the intervention groups compared to 6% in the control group. Coverage increased from 51.2% to 76.0% in strategy i); from 47.4% to 79.0% in strategy ii) and from 44.5% to 74.6% in strategy iii). Lack of information about the relevance of screening was the most important factor for not attending the screening program.
Conclusions: The study confirms that actively contacting women and including a date for a screening visit, notably increased participation in the screening program. Efforts to improve health education in preventative activities are warranted.
Trial registration: Clinical Trials.gov Identifier NCT01373723. Registered 14 June 2011.
Figures
References
-
- Globocan 2008: Estimated cancer Incidence, Mortality, Prevalence and Disability-ajusted life years (DALYs) Worldwide in 2008. http://globocan.iarc.fr/
-
- De Sanjose S, Garcia AM. Virus del papiloma humano y cancer: epidemiologia y prevencion. Monography. Madrid: Sociedad Española de Epidemiologia; 2006. pp. 1–144.
-
- Muñoz N, Castellsague X, de Gonzalez AB, Gissmann L. Chapter 1: HPV in the etiology of human cancer. Vaccine. 2006;24(Suppl 3):S3/1–10. - PubMed
-
- Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ, Muñoz N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–19. - PubMed
-
- Acera A, Rodriguez A, Trapero-Bertran M, Soteras P, Sanchez N, Bonet JM, Manresa JM, Hidalgo P, Toran P, Prieto G. Economic evaluation of three populational screening strategies for cervical cancer in the county of Valles Occidental: CRICERVA clinical trial. BMC Health Serv Res. 2011;11:278. - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous