A cationic nanoemulsion for the delivery of next-generation RNA vaccines
- PMID: 25027661
- PMCID: PMC4429691
- DOI: 10.1038/mt.2014.133
A cationic nanoemulsion for the delivery of next-generation RNA vaccines
Abstract
Nucleic acid-based vaccines such as viral vectors, plasmid DNA, and mRNA are being developed as a means to address a number of unmet medical needs that current vaccine technologies have been unable to address. Here, we describe a cationic nanoemulsion (CNE) delivery system developed to deliver a self-amplifying mRNA vaccine. This nonviral delivery system is based on Novartis's proprietary adjuvant MF59, which has an established clinical safety profile and is well tolerated in children, adults, and the elderly. We show that nonviral delivery of a 9 kb self-amplifying mRNA elicits potent immune responses in mice, rats, rabbits, and nonhuman primates comparable to a viral delivery technology, and demonstrate that, relatively low doses (75 µg) induce antibody and T-cell responses in primates. We also show the CNE-delivered self-amplifying mRNA enhances the local immune environment through recruitment of immune cells similar to an MF59 adjuvanted subunit vaccine. Lastly, we show that the site of protein expression within the muscle and magnitude of protein expression is similar to a viral vector. Given the demonstration that self-amplifying mRNA delivered using a CNE is well tolerated and immunogenic in a variety of animal models, we are optimistic about the prospects for this technology.
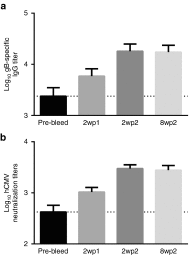
Figures







References
-
- Geall AJ, Mandl CW, Ulmer JB. RNA: The new revolution in nucleic acid vaccines. Seminars in Immunol. 2013;25:152–159. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

