Toxoplasma gondii ingests and digests host cytosolic proteins
- PMID: 25028423
- PMCID: PMC4161261
- DOI: 10.1128/mBio.01188-14
Toxoplasma gondii ingests and digests host cytosolic proteins
Abstract
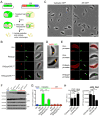
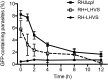
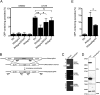
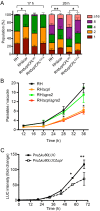
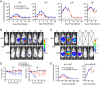
The protozoan parasite Toxoplasma gondii resides within a nonfusogenic vacuole during intracellular replication. Although the limiting membrane of this vacuole provides a protective barrier to acidification and degradation by lysosomal hydrolases, it also physically segregates the parasite from the host cytosol. Accordingly, it has been suggested that T. gondii acquires material from the host via membrane channels or transporters. The ability of the parasite to internalize macromolecules via endocytosis during intracellular replication has not been tested. Here, we show that Toxoplasma ingests host cytosolic proteins and digests them using cathepsin L and other proteases within its endolysosomal system. Ingestion was reduced in mutant parasites lacking an intravacuolar network of tubular membranes, implicating this apparatus as a possible conduit for trafficking to the parasite. Genetic ablation of proteins involved in the pathway is associated with diminished parasite replication and virulence attenuation. We show that both virulent type I and avirulent type II strain parasites ingest and digest host-derived protein, indicating that the pathway is not restricted to highly virulent strains. The findings provide the first definitive evidence that T. gondii internalizes proteins from the host during intracellular residence and suggest that protein digestion within the endolysosomal system of the parasite contributes to toxoplasmosis. Importance: Toxoplasma gondii causes significant disease in individuals with weak immune systems. Treatment options for this infection have drawbacks, creating a need to understand how this parasite survives within the cells it infects as a prelude to interrupting its survival strategies. This study reveals that T. gondii internalizes proteins from the cytoplasm of the cells it infects and degrades such proteins within a digestive compartment within the parasite. Disruption of proteins involved in the pathway reduced parasite replication and lessened disease severity. The identification of a novel parasite ingestion pathway opens opportunities to interfere with this process and improve the outcome of infection.
Copyright © 2014 Dou et al.
Figures

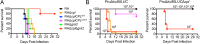
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

