The oncometabolite fumarate promotes pseudohypoxia through noncanonical activation of NF-κB signaling
- PMID: 25028521
- PMCID: PMC4148891
- DOI: 10.1074/jbc.M114.568162
The oncometabolite fumarate promotes pseudohypoxia through noncanonical activation of NF-κB signaling
Abstract
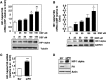
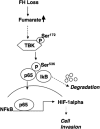
Inactivating mutations of the gene encoding the tricarboxylic acid cycle enzyme fumarate hydratase (FH) have been linked to an aggressive variant of hereditary kidney cancer (hereditary leiomyomatosis and renal cell cancer). These tumors accumulate markedly elevated levels of fumarate. Fumarate is among a growing list of oncometabolites identified in cancers with mutations of genes involved in intermediary metabolism. FH-deficient tumors are notable for their pronounced accumulation of the transcription factor hypoxia inducible factor-1α (HIF-1α) and aggressive behavior. To date, HIF-1α accumulation in hereditary leiomyomatosis and renal cell cancer tumors is thought to result from fumarate-dependent inhibition of prolyl hydroxylases and subsequent evasion from von Hippel-Lindau-dependent degradation. Here, we demonstrate a novel mechanism by which fumarate promotes HIF-1α mRNA and protein accumulation independent of the von Hippel-Lindau pathway. Here we demonstrate that fumarate promotes p65 phosphorylation and p65 accumulation at the HIF-1α promoter through non-canonical signaling via the upstream Tank binding kinase 1 (TBK1). Consistent with these data, inhibition of the TBK1/p65 axis blocks HIF-1α accumulation in cellular models of FH loss and markedly reduces cell invasion of FH-deficient RCC cancer cells. Collectively, our data demonstrate a novel mechanism by which pseudohypoxia is promoted in FH-deficient tumors and identifies TBK1 as a novel putative therapeutic target for the treatment of aggressive fumarate-driven tumors.
Keywords: Cancer Biology; Cell Metabolism; Hypoxia-inducible Factor (HIF); NF-κB (NF-KB); Small; Small Molecule.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures







References
-
- Pollard P. J., Brière J. J., Alam N. A., Barwell J., Barclay E., Wortham N. C., Hunt T., Mitchell M., Olpin S., Moat S. J., Hargreaves I. P., Heales S. J., Chung Y. L., Griffiths J. R., Dalgleish A., McGrath J. A., Gleeson M. J., Hodgson S. V., Poulsom R., Rustin P., Tomlinson I. P. (2005) Accumulation of Krebs cycle intermediates and over-expression of HIF1α in tumours which result from germline FH and SDH mutations. Hum. Mol. Genet. 14, 2231–2239 - PubMed
-
- Ward P. S., Patel J., Wise D. R., Abdel-Wahab O., Bennett B. D., Coller H. A., Cross J. R., Fantin V. R., Hedvat C. V., Perl A. E., Rabinowitz J. D., Carroll M., Su S. M., Sharp K. A., Levine R. L., Thompson C. B. (2010) The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting α-ketoglutarate to 2-hydroxyglutarate. Cancer Cell 17, 225–234 - PMC - PubMed
-
- Grubb R. L., 3rd, Franks M. E., Toro J., Middelton L., Choyke L., Fowler S., Torres-Cabala C., Glenn G. M., Choyke P., Merino M. J., Zbar B., Pinto P. A., Srinivasan R., Coleman J. A., Linehan W. M. (2007) Hereditary leiomyomatosis and renal cell cancer: a syndrome associated with an aggressive form of inherited renal cancer. J. Urol. 177, 2074–2079; discussion 2079–2080 - PubMed
-
- Tomlinson I. P., Alam N. A., Rowan A. J., Barclay E., Jaeger E. E., Kelsell D., Leigh I., Gorman P., Lamlum H., Rahman S., Roylance R. R., Olpin S., Bevan S., Barker K., Hearle N., Houlston R. S., Kiuru M., Lehtonen R., Karhu A., Vilkki S., Laiho P., Eklund C., Vierimaa O., Aittomäki K., Hietala M., Sistonen P., Paetau A., Salovaara R., Herva R., Launonen V., Aaltonen L. A., and Multiple Leiomyoma Consortium (2002) Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat. Genet. 30, 406–410 - PubMed
-
- Schofield C. J., Ratcliffe P. J. (2004) Oxygen sensing by HIF hydroxylases. Nat. Rev. Mol. Cell Biol. 5, 343–354 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

