Protective effects of Ginkgo biloba extract EGb 761 against noise trauma-induced hearing loss and tinnitus development
- PMID: 25028612
- PMCID: PMC4083883
- DOI: 10.1155/2014/427298
Protective effects of Ginkgo biloba extract EGb 761 against noise trauma-induced hearing loss and tinnitus development
Abstract
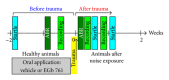
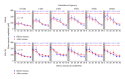
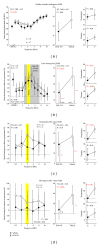
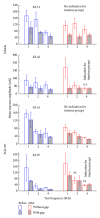
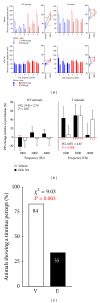
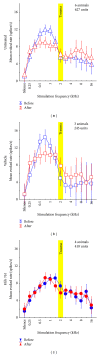
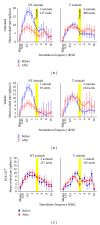
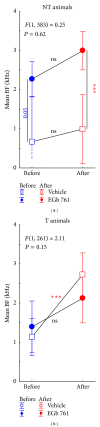
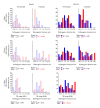
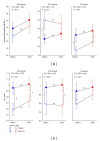
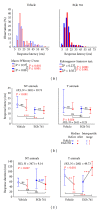
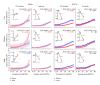
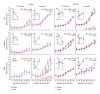
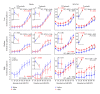
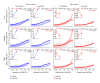
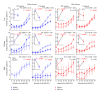
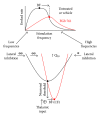
Noise-induced hearing loss (NIHL) and resulting comorbidities like subjective tinnitus are common diseases in modern societies. A substance shown to be effective against NIHL in an animal model is the Ginkgo biloba extract EGb 761. Further effects of the extract on the cellular and systemic levels of the nervous system make it a promising candidate not only for protection against NIHL but also for its secondary comorbidities like tinnitus. Following an earlier study we here tested the potential effectiveness of prophylactic EGb 761 treatment against NIHL and tinnitus development in the Mongolian gerbil. We monitored the effects of EGb 761 and noise trauma-induced changes on signal processing within the auditory system by means of behavioral and electrophysiological approaches. We found significantly reduced NIHL and tinnitus development upon EGb 761 application, compared to vehicle treated animals. These protective effects of EGb 761 were correlated with changes in auditory processing, both at peripheral and central levels. We propose a model with two main effects of EGb 761 on auditory processing, first, an increase of auditory brainstem activity leading to an increased thalamic input to the primary auditory cortex (AI) and second, an asymmetric effect on lateral inhibition in AI.
Figures

References
-
- Ruben RJ. Redefining the survival of the fittest: communication disorders in the 21st century. Laryngoscope. 2000;110(2):241–245. - PubMed
-
- Streppel M, Walger M, von Wedel H, Gaber E. Gesundheitsberichterstattung des Bundes, Statistisches Bundesamt. Heft 29 2006. Hörstörungen und Tinnitus.
-
- Katzenell U, Segal S. Hyperacusis: review and clinical guidelines. Otology and Neurotology. 2001;22(3):321–326. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

