Potential of mesenchymal stem cells in the repair of tubular injury
- PMID: 25028629
- PMCID: PMC4089627
- DOI: 10.1038/kisup.2011.21
Potential of mesenchymal stem cells in the repair of tubular injury
Abstract
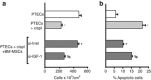
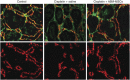
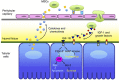
Current interventions for the treatment of acute kidney injury (AKI) are not satisfactory, and it is time to approach new strategies in order to definitely take a step forward. At its beginning, cell therapy was innovative and promising. We have shown that mesenchymal stem cells (MSCs), isolated from human and murine bone marrow (BM), behave as an efficacious tool for the treatment of cisplatin-induced AKI in mice in terms of amelioration of renal function and structure, and animal survival. Although the mechanism has not been completely elucidated, we have provided data showing that BM-MSC-mediated renal recovery involves the release at the site of injury of the growth factor, insulin-like growth factor-1. Several biological effects have been observed in renal tissues of mice treated with BM-MSCs, including increased cell proliferation, hemodynamic changes, and cell apoptosis reduction. In the same experimental model, we have tested the effect of MSCs isolated from cord blood (CB-MSCs), which, similar to BM-MSCs, not only ameliorated renal function but also protected animals from death to a remarkably higher extent. Animals receiving CB-MSCs showed reduction of oxidative stress and activation of AKT prosurvival pathway in tubular cells. These results hold great promise for future studies in patients with AKI.
Keywords: acute renal failure; kidney regeneration; mesenchymal stem cells.
Figures
Similar articles
-
Bone marrow-derived mesenchymal stem cells protect against cisplatin-induced acute kidney injury in rats by inhibiting cell apoptosis.Int J Mol Med. 2013 Dec;32(6):1262-72. doi: 10.3892/ijmm.2013.1517. Epub 2013 Oct 8. Int J Mol Med. 2013. PMID: 24126885 Free PMC article.
-
Life-sparing effect of human cord blood-mesenchymal stem cells in experimental acute kidney injury.Stem Cells. 2010 Mar 31;28(3):513-22. doi: 10.1002/stem.293. Stem Cells. 2010. PMID: 20049901
-
Mesenchymal Stem Cells: a Promising Therapeutic Tool for Acute Kidney Injury.Appl Biochem Biotechnol. 2019 Sep;189(1):284-304. doi: 10.1007/s12010-019-02995-2. Epub 2019 Apr 12. Appl Biochem Biotechnol. 2019. PMID: 30976980
-
Regenerative pharmacology for the treatment of acute kidney injury: Skeletal muscle stem/progenitor cells for renal regeneration?Pharmacol Res. 2016 Nov;113(Pt B):802-807. doi: 10.1016/j.phrs.2016.03.014. Epub 2016 Mar 18. Pharmacol Res. 2016. PMID: 27001227 Review.
-
Potential advantages of acute kidney injury management by mesenchymal stem cells.World J Stem Cells. 2014 Nov 26;6(5):644-50. doi: 10.4252/wjsc.v6.i5.644. World J Stem Cells. 2014. PMID: 25426262 Free PMC article. Review.
Cited by
-
Mesenchymal Stem Cell Therapy in Kidney Diseases: Potential and Challenges.Cell Transplant. 2023 Jan-Dec;32:9636897231164251. doi: 10.1177/09636897231164251. Cell Transplant. 2023. PMID: 37013255 Free PMC article. Review.
-
Mesenchymal Stem Cell-Derived Extracellular Vesicles: A Potential Therapeutic Strategy for Acute Kidney Injury.Front Immunol. 2021 Jun 3;12:684496. doi: 10.3389/fimmu.2021.684496. eCollection 2021. Front Immunol. 2021. PMID: 34149726 Free PMC article. Review.
-
Improved Protective Effect of Umbilical Cord Stem Cell Transplantation on Cisplatin-Induced Kidney Injury in Mice Pretreated with Antithymocyte Globulin.Stem Cells Int. 2016;2016:3585362. doi: 10.1155/2016/3585362. Epub 2016 Jan 5. Stem Cells Int. 2016. PMID: 26880955 Free PMC article.
-
Targeting Murine Mesenchymal Stem Cells to Kidney Injury Molecule-1 Improves Their Therapeutic Efficacy in Chronic Ischemic Kidney Injury.Stem Cells Transl Med. 2018 May;7(5):394-403. doi: 10.1002/sctm.17-0186. Epub 2018 Feb 15. Stem Cells Transl Med. 2018. PMID: 29446551 Free PMC article.
-
Advances in the Treatment of Kidney Disorders using Mesenchymal Stem Cells.Curr Pharm Des. 2024;30(11):825-840. doi: 10.2174/0113816128296105240305110312. Curr Pharm Des. 2024. PMID: 38482624 Review.
References
-
- Mitsiadis TA, Barrandon O, Rochat A, et al. Stem cell niches in mammals. Exp Cell Res. 2007;313:3377–3385. - PubMed
-
- Sacchetti B, Funari A, Michienzi S, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324–336. - PubMed
-
- Poulsom R, Forbes SJ, Hodivala-Dilke K, et al. Bone marrow contributes to renal parenchymal turnover and regeneration. J Pathol. 2001;195:229–235. - PubMed
-
- Morigi M, Imberti B, Zoja C, et al. Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure. J Am Soc Nephrol. 2004;15:1794–1804. - PubMed
-
- Imberti B, Morigi M, Tomasoni S, et al. Insulin-like growth factor-1 sustains stem cell-mediated renal recovery following an acute injury. J Am Soc Nephrol. 2007;18:2921–2928. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

