Radiologic-pathologic analysis of contrast-enhanced and diffusion-weighted MR imaging in patients with HCC after TACE: diagnostic accuracy of 3D quantitative image analysis
- PMID: 25028783
- PMCID: PMC4263418
- DOI: 10.1148/radiol.14140033
Radiologic-pathologic analysis of contrast-enhanced and diffusion-weighted MR imaging in patients with HCC after TACE: diagnostic accuracy of 3D quantitative image analysis
Abstract
Purpose: To evaluate the diagnostic performance of three-dimensional ( 3D three-dimensional ) quantitative enhancement-based and diffusion-weighted volumetric magnetic resonance (MR) imaging assessment of hepatocellular carcinoma ( HCC hepatocellular carcinoma ) lesions in determining the extent of pathologic tumor necrosis after transarterial chemoembolization ( TACE transarterial chemoembolization ).
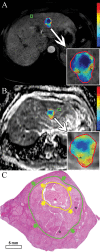
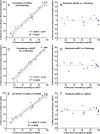
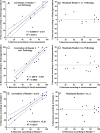
Materials and methods: This institutional review board-approved retrospective study included 17 patients with HCC hepatocellular carcinoma who underwent TACE transarterial chemoembolization before surgery. Semiautomatic 3D three-dimensional volumetric segmentation of target lesions was performed at the last MR examination before orthotopic liver transplantation or surgical resection. The amount of necrotic tumor tissue on contrast material-enhanced arterial phase MR images and the amount of diffusion-restricted tumor tissue on apparent diffusion coefficient ( ADC apparent diffusion coefficient ) maps were expressed as a percentage of the total tumor volume. Visual assessment of the extent of tumor necrosis and tumor response according to European Association for the Study of the Liver ( EASL European Association for the Study of the Liver ) criteria was performed. Pathologic tumor necrosis was quantified by using slide-by-slide segmentation. Correlation analysis was performed to evaluate the predictive values of the radiologic techniques.
Results: At histopathologic examination, the mean percentage of tumor necrosis was 70% (range, 10%-100%). Both 3D three-dimensional quantitative techniques demonstrated a strong correlation with tumor necrosis at pathologic examination (R(2) = 0.9657 and R(2) = 0.9662 for quantitative EASL European Association for the Study of the Liver and quantitative ADC apparent diffusion coefficient , respectively) and a strong intermethod agreement (R(2) = 0.9585). Both methods showed a significantly lower discrepancy with pathologically measured necrosis (residual standard error [ RSE residual standard error ] = 6.38 and 6.33 for quantitative EASL European Association for the Study of the Liver and quantitative ADC apparent diffusion coefficient , respectively), when compared with non- 3D three-dimensional techniques ( RSE residual standard error = 12.18 for visual assessment).
Conclusion: This radiologic-pathologic correlation study demonstrates the diagnostic accuracy of 3D three-dimensional quantitative MR imaging techniques in identifying pathologically measured tumor necrosis in HCC hepatocellular carcinoma lesions treated with TACE transarterial chemoembolization .
© RSNA, 2014 Online supplemental material is available for this article.
Figures


References
-
- Bruix J, Sherman M, Llovet JM, et al. . Clinical management of hepatocellular carcinoma: conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol 2001;35(3):421–430. - PubMed
-
- Lencioni R, Crocetti L. Local-regional treatment of hepatocellular carcinoma. Radiology 2012;262(1):43–58. - PubMed
-
- Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet 2012;379(9822):1245–1255. - PubMed
-
- European Association for the Study of the Liver; European Organisation for Research and Treatment of Cancer . EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56(4):908–943. - PubMed
-
- Chapiro J, Tacher V, Geschwind JF. Intraarterial therapies for primary liver cancer: state of the art. Expert Rev Anticancer Ther 2013;13(10):1157–1167. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

