Conformational mechanism for the stability of microtubule-kinetochore attachments
- PMID: 25028871
- PMCID: PMC4104050
- DOI: 10.1016/j.bpj.2014.06.004
Conformational mechanism for the stability of microtubule-kinetochore attachments
Abstract
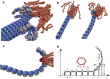
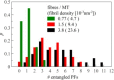
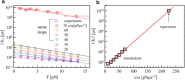
Regulating the stability of microtubule (MT)-kinetochore attachments is fundamental to avoiding mitotic errors and ensuring proper chromosome segregation during cell division. Although biochemical factors involved in this process have been identified, their mechanics still need to be better understood. Here we introduce and simulate a mechanical model of MT-kinetochore interactions in which the stability of the attachment is ruled by the geometrical conformations of curling MT-protofilaments entangled in kinetochore fibrils. The model allows us to reproduce, with good accuracy, in vitro experimental measurements of the detachment times of yeast kinetochores from MTs under external pulling forces. Numerical simulations suggest that geometrical features of MT-protofilaments may play an important role in the switch between stable and unstable attachments.
Copyright © 2014 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Cheeseman I.M., Desai A. Molecular architecture of the kinetochore-microtubule interface. Nat. Rev. Mol. Cell Biol. 2008;9:33–46. - PubMed
-
- Tanaka T.U., Rachidi N., Nasmyth K. Evidence that the Ipl1-Sli15 (Aurora kinase-INCENP) complex promotes chromosome bi-orientation by altering kinetochore-spindle pole connections. Cell. 2002;108:317–329. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

