A novel tumor necrosis factor-mediated mechanism of direct epithelial sodium channel activation
- PMID: 25029038
- PMCID: PMC4214088
- DOI: 10.1164/rccm.201405-0833OC
A novel tumor necrosis factor-mediated mechanism of direct epithelial sodium channel activation
Abstract
Rationale: Alveolar liquid clearance is regulated by Na(+) uptake through the apically expressed epithelial sodium channel (ENaC) and basolaterally localized Na(+)-K(+)-ATPase in type II alveolar epithelial cells. Dysfunction of these Na(+) transporters during pulmonary inflammation can contribute to pulmonary edema.
Objectives: In this study, we sought to determine the precise mechanism by which the TIP peptide, mimicking the lectin-like domain of tumor necrosis factor (TNF), stimulates Na(+) uptake in a homologous cell system in the presence or absence of the bacterial toxin pneumolysin (PLY).
Methods: We used a combined biochemical, electrophysiological, and molecular biological in vitro approach and assessed the physiological relevance of the lectin-like domain of TNF in alveolar liquid clearance in vivo by generating triple-mutant TNF knock-in mice that express a mutant TNF with deficient Na(+) uptake stimulatory activity.
Measurements and main results: TIP peptide directly activates ENaC, but not the Na(+)-K(+)-ATPase, upon binding to the carboxy-terminal domain of the α subunit of the channel. In the presence of PLY, a mediator of pneumococcal-induced pulmonary edema, this binding stabilizes the ENaC-PIP2-MARCKS complex, which is necessary for the open probability conformation of the channel and preserves ENaC-α protein expression, by means of blunting the protein kinase C-α pathway. Triple-mutant TNF knock-in mice are more prone than wild-type mice to develop edema with low-dose intratracheal PLY, correlating with reduced pulmonary ENaC-α subunit expression.
Conclusions: These results demonstrate a novel TNF-mediated mechanism of direct ENaC activation and indicate a physiological role for the lectin-like domain of TNF in the resolution of alveolar edema during inflammation.
Keywords: epithelial sodium channel; pneumonia; protein kinase C-α; pulmonary edema; tumor necrosis factor.
Figures


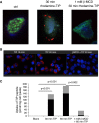
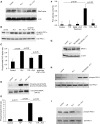


Comment in
-
Paradoxical use of tumor necrosis factor in treating pulmonary edema.Am J Respir Crit Care Med. 2014 Sep 15;190(6):595-6. doi: 10.1164/rccm.201407-1364ED. Am J Respir Crit Care Med. 2014. PMID: 25221873 No abstract available.
Similar articles
-
The Lectin-like Domain of TNF Increases ENaC Open Probability through a Novel Site at the Interface between the Second Transmembrane and C-terminal Domains of the α-Subunit.J Biol Chem. 2016 Nov 4;291(45):23440-23451. doi: 10.1074/jbc.M116.718163. Epub 2016 Sep 19. J Biol Chem. 2016. PMID: 27645999 Free PMC article.
-
Paradoxical use of tumor necrosis factor in treating pulmonary edema.Am J Respir Crit Care Med. 2014 Sep 15;190(6):595-6. doi: 10.1164/rccm.201407-1364ED. Am J Respir Crit Care Med. 2014. PMID: 25221873 No abstract available.
-
Lipoxin A(4) activates alveolar epithelial sodium channel, Na,K-ATPase, and increases alveolar fluid clearance.Am J Respir Cell Mol Biol. 2013 May;48(5):610-8. doi: 10.1165/rcmb.2012-0274OC. Am J Respir Cell Mol Biol. 2013. PMID: 23470626
-
Importance of ENaC-mediated sodium transport in alveolar fluid clearance using genetically-engineered mice.Cell Physiol Biochem. 2010;25(1):63-70. doi: 10.1159/000272051. Epub 2009 Dec 22. Cell Physiol Biochem. 2010. PMID: 20054145 Review.
-
Mini-review: novel therapeutic strategies to blunt actions of pneumolysin in the lungs.Toxins (Basel). 2013 Jul 15;5(7):1244-60. doi: 10.3390/toxins5071244. Toxins (Basel). 2013. PMID: 23860351 Free PMC article. Review.
Cited by
-
Inhalation therapy with the synthetic TIP-like peptide AP318 attenuates pulmonary inflammation in a porcine sepsis model.BMC Pulm Med. 2015 Feb 7;15:7. doi: 10.1186/s12890-015-0002-6. BMC Pulm Med. 2015. PMID: 25879802 Free PMC article.
-
Direct endothelial ENaC activation mitigates vasculopathy induced by SARS-CoV2 spike protein.Front Immunol. 2023 Aug 10;14:1241448. doi: 10.3389/fimmu.2023.1241448. eCollection 2023. Front Immunol. 2023. PMID: 37638055 Free PMC article.
-
Ion channels of the lung and their role in disease pathogenesis.Am J Physiol Lung Cell Mol Physiol. 2017 Nov 1;313(5):L859-L872. doi: 10.1152/ajplung.00285.2017. Epub 2017 Oct 12. Am J Physiol Lung Cell Mol Physiol. 2017. PMID: 29025712 Free PMC article. Review.
-
The Lectin-like Domain of TNF Increases ENaC Open Probability through a Novel Site at the Interface between the Second Transmembrane and C-terminal Domains of the α-Subunit.J Biol Chem. 2016 Nov 4;291(45):23440-23451. doi: 10.1074/jbc.M116.718163. Epub 2016 Sep 19. J Biol Chem. 2016. PMID: 27645999 Free PMC article.
-
The TNF-derived TIP peptide activates the epithelial sodium channel and ameliorates experimental nephrotoxic serum nephritis.Kidney Int. 2019 Jun;95(6):1359-1372. doi: 10.1016/j.kint.2018.12.022. Epub 2019 Mar 21. Kidney Int. 2019. PMID: 30905471 Free PMC article.
References
-
- Ware LB, Matthay MA. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;163:1376–1383. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

