Randomized controlled trial of calcitriol in severe sepsis
- PMID: 25029202
- PMCID: PMC4214090
- DOI: 10.1164/rccm.201405-0988OC
Randomized controlled trial of calcitriol in severe sepsis
Abstract
Rationale: Vitamin D and its metabolites have potent immunomodulatory effects in vitro, including up-regulation of cathelicidin, a critical antimicrobial protein.
Objectives: We investigated whether administration of 1,25-dihydroxyvitamin D (calcitriol) to critically ill patients with sepsis would have beneficial effects on markers of innate immunity, inflammation, and kidney injury.
Methods: We performed a double-blind, randomized, placebo-controlled, physiologic study among 67 critically ill patients with severe sepsis or septic shock. Patients were randomized to receive a single dose of calcitriol (2 μg intravenously) versus placebo. The primary outcome was plasma cathelicidin protein levels assessed 24 hours after study drug administration. Secondary outcomes included leukocyte cathelicidin mRNA expression, plasma cytokine levels (IL-10, IL-6, tumor necrosis factor-α, IL-1β, and IL-2), and urinary kidney injury markers.
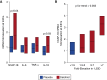
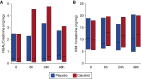
Measurements and main results: Patients randomized to calcitriol (n = 36) versus placebo (n = 31) had similar plasma cathelicidin protein levels at 24 hours (P = 0.16). Calcitriol-treated patients had higher cathelicidin (P = 0.04) and IL-10 (P = 0.03) mRNA expression than placebo-treated patients 24 hours after study drug administration. Plasma cytokine levels (IL-10, IL-6, tumor necrosis factor-α, IL-1β, and IL-2) and urinary kidney injury markers were similar in calcitriol- versus placebo-treated patients (P > 0.05 for all comparisons). Calcitriol had no effect on clinical outcomes nor were any adverse effects observed.
Conclusions: Calcitriol administration did not increase plasma cathelicidin protein levels in critically ill patients with sepsis and had mixed effects on other immunomodulatory markers. Additional phase II trials investigating the dose and timing of calcitriol as a therapeutic agent in specific sepsis phenotypes may be warranted. Clinical trial registered with www.clinicaltrials.gov (NCT 01689441).
Trial registration: ClinicalTrials.gov NCT01689441.
Keywords: 1,25-dihydroxyvitamin D; cathelicidin; critical illness; innate immunity; vitamin D.
Figures


Comment in
-
Vitamin D supplementation in sepsis and critical illness: where are we now?Am J Respir Crit Care Med. 2014 Sep 1;190(5):483-5. doi: 10.1164/rccm.201408-1443ED. Am J Respir Crit Care Med. 2014. PMID: 25171307 Free PMC article. No abstract available.
-
Native and active vitamin D in intensive care: who and how we treat is crucially important.Am J Respir Crit Care Med. 2014 Nov 15;190(10):1193-4. doi: 10.1164/rccm.201407-1354LE. Am J Respir Crit Care Med. 2014. PMID: 25398112 No abstract available.
-
Reply: active and native vitamin D in critical illness.Am J Respir Crit Care Med. 2014 Nov 15;190(10):1194-6. doi: 10.1164/rccm.201408-1454LE. Am J Respir Crit Care Med. 2014. PMID: 25398113 Free PMC article. No abstract available.
-
A few follow-up questions to a recent calcitriol and sepsis study.Am J Respir Crit Care Med. 2014 Nov 15;190(10):1194. doi: 10.1164/rccm.201409-1696LE. Am J Respir Crit Care Med. 2014. PMID: 25398114 No abstract available.
References
-
- Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369:840–851. - PubMed
-
- Braun AB, Litonjua AA, Moromizato T, Gibbons FK, Giovannucci E, Christopher KB. Association of low serum 25-hydroxyvitamin D levels and acute kidney injury in the critically ill. Crit Care Med. 2012;40:3170–3179. - PubMed
-
- Ginde AA, Camargo CA, Jr, Shapiro NI. Vitamin D insufficiency and sepsis severity in emergency department patients with suspected infection. Acad Emerg Med. 2011;18:551–554. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

