Activated STING in a vascular and pulmonary syndrome
- PMID: 25029335
- PMCID: PMC4174543
- DOI: 10.1056/NEJMoa1312625
Activated STING in a vascular and pulmonary syndrome
Abstract
Background: The study of autoinflammatory diseases has uncovered mechanisms underlying cytokine dysregulation and inflammation.
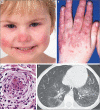
Methods: We analyzed the DNA of an index patient with early-onset systemic inflammation, cutaneous vasculopathy, and pulmonary inflammation. We sequenced a candidate gene, TMEM173, encoding the stimulator of interferon genes (STING), in this patient and in five unrelated children with similar clinical phenotypes. Four children were evaluated clinically and immunologically. With the STING ligand cyclic guanosine monophosphate-adenosine monophosphate (cGAMP), we stimulated peripheral-blood mononuclear cells and fibroblasts from patients and controls, as well as commercially obtained endothelial cells, and then assayed transcription of IFNB1, the gene encoding interferon-β, in the stimulated cells. We analyzed IFNB1 reporter levels in HEK293T cells cotransfected with mutant or nonmutant STING constructs. Mutant STING leads to increased phosphorylation of signal transducer and activator of transcription 1 (STAT1), so we tested the effect of Janus kinase (JAK) inhibitors on STAT1 phosphorylation in lymphocytes from the affected children and controls.
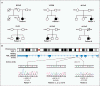
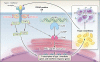
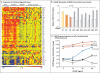
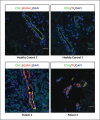
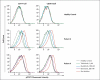
Results: We identified three mutations in exon 5 of TMEM173 in the six patients. Elevated transcription of IFNB1 and other gene targets of STING in peripheral-blood mononuclear cells from the patients indicated constitutive activation of the pathway that cannot be further up-regulated with stimulation. On stimulation with cGAMP, fibroblasts from the patients showed increased transcription of IFNB1 but not of the genes encoding interleukin-1 (IL1), interleukin-6 (IL6), or tumor necrosis factor (TNF). HEK293T cells transfected with mutant constructs show elevated IFNB1 reporter levels. STING is expressed in endothelial cells, and exposure of these cells to cGAMP resulted in endothelial activation and apoptosis. Constitutive up-regulation of phosphorylated STAT1 in patients' lymphocytes was reduced by JAK inhibitors.
Conclusions: STING-associated vasculopathy with onset in infancy (SAVI) is an autoinflammatory disease caused by gain-of-function mutations in TMEM173. (Funded by the Intramural Research Program of the National Institute of Arthritis and Musculoskeletal and Skin Diseases; ClinicalTrials.gov number, NCT00059748.).
Figures
Comment in
-
STING-associated vasculopathy with onset in infancy--a new interferonopathy.N Engl J Med. 2014 Aug 7;371(6):568-71. doi: 10.1056/NEJMe1407246. Epub 2014 Jul 16. N Engl J Med. 2014. PMID: 25029336 No abstract available.
-
Autoinflammation: A new STING-associated monogenic autoinflammatory disease.Nat Rev Rheumatol. 2014 Sep;10(9):512. doi: 10.1038/nrrheum.2014.126. Epub 2014 Aug 5. Nat Rev Rheumatol. 2014. PMID: 25090944 No abstract available.
-
[Early aggressive systemic inflammatory vascular diseases: mutations in adenosine desaminase 2 and interferonopathies].Ann Dermatol Venereol. 2014 Dec;141(12):808-9. doi: 10.1016/j.annder.2014.10.006. Epub 2014 Nov 1. Ann Dermatol Venereol. 2014. PMID: 25433938 French. No abstract available.
References
-
- Crow YJ. Aicardi-Goutières syndrome. Handb Clin Neurol. 2013;113:1629–35. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
