A novel genomic signature with translational significance for human idiopathic pulmonary fibrosis
- PMID: 25029475
- PMCID: PMC4370242
- DOI: 10.1165/rcmb.2013-0310OC
A novel genomic signature with translational significance for human idiopathic pulmonary fibrosis
Abstract
The bleomycin-induced rodent lung fibrosis model is commonly used to study mechanisms of lung fibrosis and to test potential therapeutic interventions, despite the well recognized dissimilarities to human idiopathic pulmonary fibrosis (IPF). Therefore, in this study, we sought to identify genomic commonalities between the gene expression profiles from 100 IPF lungs and 108 control lungs that were obtained from the Lung Tissue Research Consortium, and rat lungs harvested at Days 3, 7, 14, 21, 28, 42, and 56 after bleomycin instillation. Surprisingly, the highest gene expression similarity between bleomycin-treated rat and IPF lungs was observed at Day 7. At this point of maximal rat-human commonality, we identified a novel set of 12 disease-relevant translational gene markers (C6, CTHRC1, CTSE, FHL2, GAL, GREM1, LCN2, MMP7, NELL1, PCSK1, PLA2G2A, and SLC2A5) that was able to separate almost all patients with IPF from control subjects in our cohort and in two additional IPF/control cohorts (GSE10667 and GSE24206). Furthermore, in combination with diffusing capacity of carbon monoxide measurements, four members of the translational gene marker set contributed to stratify patients with IPF according to disease severity. Significantly, pirfenidone attenuated the expression change of one (CTHRC1) translational gene marker in the bleomycin-induced lung fibrosis model, in transforming growth factor-β1-treated primary human lung fibroblasts and transforming growth factor-β1-treated human epithelial A549 cells. Our results suggest that a strategy focused on rodent model-human disease commonalities may identify genes that could be used to predict the pharmacological impact of therapeutic interventions, and thus facilitate the development of novel treatments for this devastating lung disease.
Keywords: animal model; biomarkers; genomics; lung fibrosis.
Figures
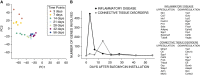
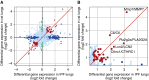
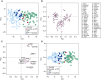

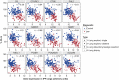
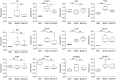
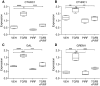
Comment in
-
The bleomycin model: in pursuit of relevant biomakers.Am J Respir Cell Mol Biol. 2015 Nov;53(5):748. doi: 10.1165/rcmb.2015-0162LE. Am J Respir Cell Mol Biol. 2015. PMID: 26517753 No abstract available.
-
Reply: the bleomycin model: in pursuit of relevant biomakers.Am J Respir Cell Mol Biol. 2015 Nov;53(5):748-9. doi: 10.1165/rcmb.2015-0196LE. Am J Respir Cell Mol Biol. 2015. PMID: 26517754 Free PMC article. No abstract available.
References
-
- Olson AL, Swigris JJ, Lezotte DC, Norris JM, Wilson CG, Brown KK. Mortality from pulmonary fibrosis increased in the United States from 1992 to 2003. Am J Respir Crit Care Med. 2007;176:277–284. - PubMed
-
- Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier J-F, Flaherty KR, Lasky JA, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. - PMC - PubMed
-
- Borchers AT, Chang C, Keen CL, Gershwin ME. Idiopathic pulmonary fibrosis—an epidemiological and pathological review. Clin Rev Allergy Immunol. 2010;40:117–134. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

