Inhaled corticosteroids in children with persistent asthma: dose-response effects on growth
- PMID: 25030199
- PMCID: PMC8932085
- DOI: 10.1002/14651858.CD009878.pub2
Inhaled corticosteroids in children with persistent asthma: dose-response effects on growth
Abstract
Background: Inhaled corticosteroids (ICS) are the first-line treatment for children with persistent asthma. Their potential for growth suppression remains a matter of concern for parents and physicians.
Objectives: To assess whether increasing the dose of ICS is associated with slower linear growth, weight gain and skeletal maturation in children with asthma.
Search methods: We searched the Cochrane Airways Group Specialised Register of trials (CAGR) and the ClinicalTrials.gov website up to March 2014.
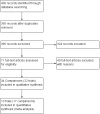
Selection criteria: Studies were eligible if they were parallel-group randomised trials evaluating the impact of different doses of the same ICS using the same device in both groups for a minimum of three months in children one to 17 years of age with persistent asthma.
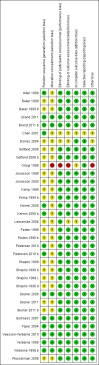
Data collection and analysis: Two review authors ascertained methodological quality independently using the Cochrane Risk of bias tool. The primary outcome was linear growth velocity. Secondary outcomes included change over time in growth velocity, height, weight, body mass index and skeletal maturation.
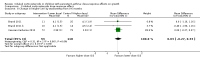
Main results: Among 22 eligible trials, 17 group comparisons were derived from 10 trials (3394 children with mild to moderate asthma), measured growth and contributed data to the meta-analysis. Trials used ICS (beclomethasone, budesonide, ciclesonide, fluticasone or mometasone) as monotherapy or as combination therapy with a long-acting beta2-agonist and generally compared low (50 to 100 μg) versus low to medium (200 μg) doses of hydrofluoroalkane (HFA)-beclomethasone equivalent over 12 to 52 weeks. In the four comparisons reporting linear growth over 12 months, a significant group difference was observed, clearly indicating lower growth velocity in the higher ICS dose group of 5.74 cm/y compared with 5.94 cm/y on lower-dose ICS (N = 728 school-aged children; mean difference (MD)0.20 cm/y, 95% confidence interval (CI) 0.02 to 0.39; high-quality evidence): No statistically significant heterogeneity was noted between trials contributing data. The ICS molecules (ciclesonide, fluticasone, mometasone) used in these four comparisons did not significantly influence the magnitude of effect (X(2) = 2.19 (2 df), P value 0.33). Subgroup analyses on age, baseline severity of airway obstruction, ICS dose and concomitant use of non-steroidal antiasthmatic drugs were not performed because of similarity across trials or inadequate reporting. A statistically significant group difference was noted in unadjusted change in height from zero to three months (nine comparisons; N = 944 children; MD 0.15, 95% CI -0.28 to -0.02; moderate-quality evidence) in favour of a higher ICS dose. No statistically significant group differences in change in height were observed at other time points, nor were such differences in weight, bone mass index and skeletal maturation reported with low quality of evidence due to imprecision.
Authors' conclusions: In prepubescent school-aged children with mild to moderate persistent asthma, a small but statistically significant group difference in growth velocity was observed between low doses of ICS and low to medium doses of HFA-beclomethasone equivalent, favouring the use of low-dose ICS. No apparent difference in the magnitude of effect was associated with three molecules reporting one-year growth velocity, namely, mometasone, ciclesonide and fluticasone. In view of prevailing parents' and physicians' concerns about the growth suppressive effect of ICS, lack of or incomplete reporting of growth velocity in more than 86% (19/22) of eligible paediatric trials, including those using beclomethasone and budesonide, is a matter of concern. All future paediatric trials comparing different doses of ICS with or without placebo should systematically document growth. Findings support use of the minimal effective ICS dose in children with asthma.
Conflict of interest statement
Aniela Ignea Pruteanu, Bhupendrasinh Chauhan, Linjie Zhang and Sílvio OM Prietsch: none known.
Prof. Francine Ducharme has received travel support, research funds and fees for speaking from Glaxo SmithKline, Novartis, Nycomed and/or Merck Frosst Inc.
Figures
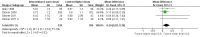

















Republished in
-
Inhaled corticosteroids in children with persistent asthma: dose-response effects on growth.Evid Based Child Health. 2014 Dec;9(4):931-1046. doi: 10.1002/ebch.1989. Evid Based Child Health. 2014. PMID: 25504973
References
References to studies included in this review
Allen 1998 {published data only}
-
- Allen DB, Bronsky EA, LaForce CF, Nathan RA, Tinkelman D, Vandewalker ML, et al. Growth in asthmatic children treated with fluticasone propionate. Journal of Pediatrics 1998;132(3 I):472‐7. - PubMed
Baker 1999 {published data only}
-
- Baker J, Mellon M, Wald J, Welch M, Cruz‐Rivera M. A multiple‐dosing, placebo‐controlled study of budesonide inhalation suspension given once or twice daily for treatment of persistent asthma in young children and infants. Pediatrics 1999;103(2):414‐21. - PubMed
Baker 1999 b {published data only}
-
- Baker J, Mellon M, Wald J, Welch M, Cruz‐Rivera M. A multiple‐dosing, placebo‐controlled study of budesonide inhalation suspension given once or twice daily for treatment of persistent asthma in young children and infants. Pediatrics 1999;103(2):414‐421. - PubMed
Brand 2011 {published data only}
-
- Brand PL, Luz Garcia‐Garcia M, Morison A, Vermeulen JH, Weber HC. Ciclesonide in wheezy preschool children with a positive asthma predictive index or atopy. Respiratory Medicine 2011;105(11):1588‐95. - PubMed
Brand 2011 b {published data only}
-
- Brand PL, Luz Garcia‐Garcia M, Morison A, Vermeulen JH, Weber HC. Ciclesonide in wheezy preschool children with a positive asthma predictive index or atopy. Respiratory Medicine 2011/11;105(11):1588‐95. - PubMed
Chen 2001 {published data only}
-
- Chen A, Chen R, Zhong N. Systemic side effects of long‐term treatment with low dose inhaled corticosteroids in children with asthma. Chinese Journal of Tuberculosis and Respiratory Diseases 2001;24(12):740‐3. - PubMed
Doniec 2004 {published data only}
-
- Doniec Z, Pierzchala‐Koziec K, Tomalak W, Nowak D, Kurzawa R. Powder budesonide decreases plasma level of native and cryptic met‐enkephalin in asthmatic children. International Review of Allergology and Clinical Immunology 2004;10(3):105‐9.
Gelfand 2006 {published data only}
-
- Gelfand EW, Georgitis JW, Noonan M, Ruff ME. Once‐daily ciclesonide in children: efficacy and safety in asthma. Journal of Pediatrics 2006;148(3):377‐83. - PubMed
Gelfand 2006 b {published data only}
-
- Gelfand EW, Georgitis JW, Noonan M, Ruff ME. Once‐daily ciclesonide in children: efficacy and safety in asthma. J Pediatrics 2006;148(3):377‐83. - PubMed
Giorgi 1998 {published data only}
-
- Giorgi PL, Oggiano N, Kantar A, Coppa GV, Ricciotti R, Arena F, et al. Bone metabolism in children with asthma treated with nebulized flunisolide: a multicenter Italian study. Current Therapeutic Research, Clinical and Experimental 1998;59(12):896‐908.
Jonasson 1998 {published data only}
-
- Jónasson G, Carlsen K‐H, Blomqvist P. Clinical efficacy of low‐dose inhaled budesonide once or twice daily in children with mild asthma not previously treated with steroids. European Respiratory Journal 1998;12:1099‐104. - PubMed
Jonasson 2000 {published data only}
-
- Jonasson G, Carlsen KH, Jonasson C, Mowinckel P. Low‐dose inhaled budesonide once or twice daily for 27 months in children with mild asthma. Allergy 2000;55(8):740‐8. - PubMed
Kemp 1999 {published data only}
-
- Kemp JP, Skoner DP, Szefler SJ, Walton‐Bowen K, Cruz‐Rivera M, Smith JA. Once‐daily budesonide inhalation suspension for the treatment of persistent asthma in infants and young children. Annals of Allergy 1999;83(3):231‐9. - PubMed
Kemp 1999 b {published data only}
-
- Kemp JP, Skoner DP, Szefler SJ, Walton‐Bowen K, Cruz‐Rivera M. Once‐daily budesonide inhalation suspension for the treatment of persistent asthma in infants and young children. Annals of Allergy 1999;83(3):231‐9. - PubMed
Kerwin 2008 {published data only}
-
- Kerwin EM, Pearlman DS, Guia T, Carlsson LG, Gillen M, Uryniak T, et al. Evaluation of efficacy and safety of budesonide delivered via two dry powder inhalers. Current Medical Research and Opinion 2008;24(5):1497‐510. - PubMed
Kerwin 2008 b {published data only}
-
- Kerwin EM, Pearlman DS, Guia T, Carlsson LG, Gillen M, Uryniak T. Evaluation of efficacy and safety of budesonide delivered via two dry powder inhalers. Current Medical Research and Opinion 2008;24(5):1497‐510. - PubMed
Lemanske 2004 {published data only}
-
- Lemanske RF, Lockey RF, Murphy KR. Effects of one year of treatment with mometasone furoate metered dose inhaler (MF‐MDI) on growth in children with asthma. European Respiratory Journal 2004;24(Suppl 48):379s.
Peden 1998 {published data only}
-
- Peden DB, Berger WE, Noonan MJ, Thomas MR, Hendricks VL, Hamedani AG. Inhaled fluticasone propionate delivered by means of two different multidose powder inhalers is effective and safe in a large pediatric population with persistent asthma. Journal of Allergy & Clinical Immunology 1998;102(1):32‐8. - PubMed
Peden 1998 b {published data only}
-
- Peden DB, Berger WE, Noonan MJ, Thomas MR, Hendricks VL, Hamedani AG. Inhaled fluticasone propionate delivered by means of two different multidose powder inhalers is effective and safe in a large pediatric population with persistent asthma. Journal of Allergy & Clinical Immunology 1998;102(1):32‐8. - PubMed
Pedersen 2010 {published data only}
-
- Pedersen S, Potter P, Dachev S, Bosheva M, Kaczmarek J, Springer E, et al. Efficacy and safety of three ciclesonide doses vs placebo in children with asthma: the rainbow study. Respiratory Medicine 2010;104(11):1618‐28. - PubMed
Pedersen 2010 b {published data only}
-
- Pedersen S, Potter P, Dachev S, Bosheva M, Kaczmarek J, Springer E, et al. Efficacy and safety of three ciclesonide doses vs placebo in children with asthma: the rainbow study. Respiratory Medicine 2010;104(11):1618‐28. - PubMed
Shapiro 1998 {published data only}
-
- Shapiro G, Bronsky EA, LaForce CF, Mendelson L, Pearlman D, Schwartz RH, et al. Dose‐related efficacy of budesonide administered via a dry powder inhaler in the treatment of children with moderate to severe persistent asthma. Journal of Pediatrics 1998;132(6):976‐82. - PubMed
Shapiro 1998 b {published data only}
-
- Shapiro G, Bronsky EA, LaForce CF, Mendelson L, Pearlman D, Schwartz RH. Dose‐related efficacy of budesonide administered via a dry powder inhaler in the treatment of children with moderate to severe persistent asthma. J Pediatrics 1998;132(6):976‐82. - PubMed
Shapiro 1998 c {published data only}
-
- Shapiro G, Bronsky EA, LaForce CF, Mendelson L, Pearlman D, Schwartz RH. Dose‐related efficacy of budesonide administered via a dry powder inhaler in the treatment of children with moderate to severe persistent asthma. J Pediatrics 1998;132(6):976‐82. - PubMed
Shapiro 1998 d {published data only}
-
- Shapiro G, Bronsky EA, LaForce CF, Mendelson L, Pearlman D, Schwartz RH. Dose‐related efficacy of budesonide administered via a dry powder inhaler in the treatment of children with moderate to severe persistent asthma. J Pediatrics 1998;132(6):976‐82. - PubMed
Skoner 2008 {published data only}
-
- Skoner DP, Maspero J, Banerji D. Assessment of the long‐term safety of inhaled ciclesonide on growth in children with asthma. Pediatrics 2008;121(1):e1‐14. - PubMed
Skoner 2011 {published data only}
-
- Skoner DP, Meltzer EO, Milgrom HA, Stryszak P, Teper A, Staudinger H. Effects of inhaled mometasone furoate on growth velocity and adrenal function: a placebo‐controlled trial in children 4‐9 years old with mild persistent asthma. Journal of Asthma 2011;48(8):848‐59. - PubMed
Skoner 2011 b {published data only}
-
- Skoner DP, Meltzer EO, Milgrom HA, Stryszak P, Teper A, Staudinger H. Effects of inhaled mometasone furoate on growth velocity and adrenal function: a placebo‐controlled trial in children 4‐9 years old with mild persistent asthma. Journal of Asthma 2011;48(8):848‐59. - PubMed
Sorkness 2007 {published data only}
-
- Sorkness CA, Lemanske RF Jr, Mauger DT, Boehmer SJ, Chinchilli VM, Martinez FD, et al. Long‐term comparison of 3 controller regimens for mild‐moderate persistent childhood asthma: the Pediatric Asthma Controller Trial. Journal of Allergy and Clinical Immunology 2007;119(1):64‐72. - PubMed
Teper 2004 {published data only}
-
- Teper AM, Colom AJ, Kofman CD, Maffey AF, Vidaurreta SM, Bergada I. Effects of inhaled fluticasone propionate in children less than 2 years old with recurrent wheezing. Pediatric Pulmonology 2004;37(2):111‐5. - PubMed
Vaessen‐Verberne 2010 {published data only}
-
- Vaessen‐Verberne AA, Berg NJ, Nierop JC, Brackel HJ, Gerrits GP, Hop WC, et al. Combination therapy salmeterol/fluticasone versus doubling dose of fluticasone in children with asthma. American Journal of Respiratory and Critical Care Medicine 2010;182(10):1221‐7. - PubMed
Verberne 1998 {published data only}
-
- Verberne AA, Frost C, Duiverman EJ, Grol MH, Kerrebijn KF. Addition of salmeterol versus doubling the dose of beclomethasone in children with asthma. The Dutch Asthma Study Group. American Journal of Respiratory and Critical Care Medicine 1998;158(1):213‐9. - PubMed
Verberne 1998 b {published data only}
-
- Verberne AA, Frost C, Duiverman EJ, Grol MH, Kerrebijn KF. Addition of salmeterol versus doubling the dose of beclomethasone in children with asthma. The Dutch Asthma Study Group. American Journal of Respiratory and Critical Care Medicine 1998;158(1):213‐9. - PubMed
Wasserman 2006 {published data only}
-
- Wasserman RL, Baker JW, Kim KT, Blake KV, Scott CA, Wu W, et al. Efficacy and safety of inhaled fluticasone propionate chlorofluorocarbon in 2‐ to 4‐year‐old patients with asthma: results of a double‐blind, placebo‐controlled study. Annals of Allergy, Asthma, and Immunology 2006;96(6):808‐18. - PubMed
References to studies excluded from this review
Agertoft 2004 {published data only}
-
- Agertoft L, Pedersen S. Inhaled ciclesonide does not effect lower leg growth rate or HPA‐axis function in children with mild asthma. European Respiratory Journal 2004;24(Suppl 48):377s.
Antoniu 2003 {published data only}
-
- Antoniu SA. The START study: when to start to treat with inhaled steroids in asthma?. Expert Review of Pharmacoeconomics & Outcomes Research 2003;3(3):223‐5. - PubMed
Apold 1975 {published data only}
-
- Apold J, Djoseland O. Inhaled beclomethasone dipropionate in the treatment of childhood asthma. Postgraduate Medical Journal 1975;51(Suppl 4):104‐5. - PubMed
Asrilant 1975 {published data only}
-
- Asrilant M. Beclomethasone dipropionate: an aerosol corticosteroid for topical use in bronchial asthma. Postgraduate Medical Journal 1975;51(Suppl 4):79‐83. - PubMed
Bateman 2008 {published data only}
-
- Bateman ED, Cheung D, Lapa e Silva J, Gohring UM, Schafer M, Engelstatter R. Randomized comparison of ciclesonide 160 and 640 mug/day in severe asthma. Pulmonary Pharmacology and Therapeutics 2008;21(3):489‐98. - PubMed
Baxter‐Jones 1998 {published data only}
-
- Baxter‐Jones AD, Helms PJ. Effect of 6 month daily treatment with inhaled corticosteroids on lower leg growth in pre‐school wheezing children: a pragmatic trial. Thorax 1998;53(Suppl 4):A5 S19.
Berger 2005 {published data only}
-
- Berger WE, Qaqundah PY, Blake K, Rodriguez‐Santana J, Irani AM, Xu J, et al. Safety of budesonide inhalation suspension in infants aged six to twelve months with mild to moderate persistent asthma or recurrent wheeze. The Journal of Pediatrics 2005;146(1):91‐5. - PubMed
Bernstein 1999 {published data only}
-
- Bernstein DI, Berkowitz RB, Chervinsky P, Dvorin DJ, Finn AF, Gross GN, et al. Dose‐ranging study of a new steroid for asthma: mometasone furoate dry powder inhaler. Respiratory Medicine 1999;93(9):603‐12. - PubMed
Birkebaek 1995 {published data only}
Breborowicz 2005 {published data only}
-
- Breborowicz A, Niedziela M. Low risk of adrenal dysfunction in children with severe asthma treated with high dose inhaled glucocorticoids. European Respiratory Journal 2005;26(Suppl 49SP):Abstract No. 1058.
Brook 1998 {published data only}
-
- Brook CG. Short stature never killed anybody. Journal of Pediatrics 1998;133(5):591‐2. - PubMed
Brown 1973 {published data only}
Chuchalin 2008 {published data only}
-
- Chuchalin A, Jacques L, Frith L. Salmeterol/fluticasone propionate via Diskus[trademark] once daily versus fluticasone propionate twice daily in patients with mild asthma not previously receiving maintenance corticosteroids. Clinical Drug Investigation 2008;28(3):169‐81. - PubMed
Dickson 1973 {published data only}
Ferguson 2002 {published data only}
-
- Ferguson AC, Bever HP, Teper AM, Lasytsya OI, Whitehead PJ. Fluticasone propionate 100µg bd (FP100) has significantly less effect than budesonide 200µg bd (BUD200) on childhood growth over 1 year of treatment in asthmatics. European Respiratory Journal 2002;20(Suppl 38):219s.
Godfrey 1973 {published data only}
Godfrey 1974 {published data only}
Guarnaccia 1996 {published data only}
-
- Guarnaccia S, Buzi F, LaGrutta S, Marini S, Laffranchi MG, Brunori A, et al. High dose inhaled corticosteroids (IC) in children with asthma: influence on bone metabolism. European Respiratory Journal 1996;9(Suppl 23):295s.
Guo 2002 {published data only}
-
- Guo JG, Cheng ST. The efficacy of low‐dose oral aminophylline combined with inhaled corticosteroid in the treatment of asthmatic children in remission period. Acta Academic Medicine Xuzhou 2002;22(4):349‐51.
Gwynn 1977 {published data only}
Hansel 2006 {published data only}
-
- Hansel TT, Benezet O, Kafe H, Ponitz HH, Cheung D, Engelstatter R, et al. A multinational, 12‐week, randomized study comparing the efficacy and tolerability of ciclesonide and budesonide in patients with asthma. Clinical Therapeutics 2006;28(6):906‐20. - PubMed
Kaiser 2008 {published data only}
-
- Kaiser H, Parasuraman B, Boggs R, Miller CJ, Leidy NK, O'Dowd L. Onset of effect of budesonide and formoterol administered via one pressurized metered‐dose inhaler in patients with asthma previously treated with inhaled corticosteroids. Annals of Allergy, Asthma, and Immunology 2008;101(3):295‐303. - PubMed
Karpel 2007 {published data only}
-
- Karpel JP, Nayak A, Lumry W, Craig TJ, Kerwin E, Fish JE, et al. Inhaled mometasone furoate reduces oral prednisone usage and improves lung function in severe persistent asthma. Respiratory Medicine 2007;101(3):628‐37. - PubMed
Kemp 2004 {published data only}
-
- Kemp JP, Osur S, Shrewsbury SB, Herje NE, Duke SP, Harding SM, et al. Potential effects of fluticasone propionate on bone mineral density in patients with asthma: a 2‐year randomized, double‐blind, placebo‐controlled trial. Mayo Clinic Proceedings 2004;79(4):458‐66. - PubMed
Lang 2013 {published data only}
-
- Lang JE, Dozor AJ, Holbrook JT, Mougey E, Krishnan S, Sweeten S, et al. Biologic mechanisms of environmental tobacco smoke in children with poorly controlled asthma: results from a multicenter clinical trial. Journal of Allergy and Clinical Immunology: In Practice 2013;1(2):172‐180.e2. - PMC - PubMed
Laursen 1986 {published data only}
-
- Laursen LC, Taudorf E, Weeke B. High‐dose inhaled budesonide in treatment of severe steroid‐dependent asthma. European Journal of Respiratory Diseases 1986;68(1):19‐28. - PubMed
Lipworth 1996 {published data only}
-
- Lipworth BJ, Clark D. High‐dose inhaled steroids in asthmatic children. Lancet 1996;348(9030):820; discussion 821. - PubMed
Lovera 1975 {published data only}
-
- Lovera J, Collins‐Williams C, Bailey J. Beclomethasone dipropionate by aerosol in the treatment in asthmatic children. Postgraduate Medical Journal 1975;51(Suppl 4):96‐8. - PubMed
McAllen 1974 {published data only}
Neffen 2006 {published data only}
-
- Neffen H, Ruff M, Zhang P, Lloyd M, Banjeri D. Ciclesonide administered once daily has no effect on skeletal maturity in prepubertal children with mild persistent asthma [Abstract]. Journal of Allergy and Clinical Immunology 2006;117(2):184.
Nelson 2000 {published data only}
-
- Nelson HS, Kane RE, Petillo J, Banerji D, Anolik R, Bosso J, et al. Long‐term safety of a non‐chlorofluorocarbon‐containing triamcinolone acetonide inhalation aerosol in patients with asthma. Journal of Asthma 2000;37(2):145‐52. - PubMed
Niu 1998 {published data only}
-
- Niu CK, Huang SC, Huang CB. Effect of short‐course budesonide on the bone turnover of asthmatic children. Pediatric Pulmonology 1998;26(4):290‐2. - PubMed
Otsuki 2009 {published data only}
Pearlman 2005 {published data only}
-
- Pearlman DS, Berger WE, Kerwin E, LaForce C, Kundu S, Banerji D. Once‐daily ciclesonide improves lung function and is well tolerated by patients with mild‐to‐moderate persistent asthma. Journal of Allergy and Clinical Immunology 2005;116(6):1206‐12. - PubMed
Pedeersen 2003 {published data only}
-
- Pedeersen S, Agertoft L, Lee T, Staudinger H. Lower‐leg growth in children with asthma during treatment with inhaled corticosteroids. Journal of Allergy and Clinical Immunology 2003;111(2 Suppl):S269.
Pedersen 2002 {published data only}
-
- Pedersen S, Warner J, Wahn U, Staab D, Bourgeois M, Essen‐Zandvliet E, et al. Growth, systemic safety, and efficacy during 1 year of asthma treatment with different beclomethasone dipropionate formulations: an open‐label, randomized comparison of extrafine and conventional aerosols in children. Pediatrics 2002;109(6):e92. - PubMed
Peroni 2005 {published data only}
-
- Peroni DG, Piacentini GL, Bodini A, Ress M, Costella S, Boner AL. Montelukast versus formoterol as second‐line therapy in asthmatic children exposed to relevant allergens. Allergy and Asthma Proceedings 2005;26(4):283‐6. - PubMed
Phipatanakul 2003 {published data only}
-
- Phipatanakul W, Greene C, Downes SJ, Cronin B, Eller TJ, Schneider LC, et al. Montelukast improves asthma control in asthmatic children maintained on inhaled corticosteroids. Annals of Allergy, Asthma, and Immunology 2003;91(1):49‐54. - PubMed
Pines 1973 {published data only}
-
- Pines A. Beclomethasone dipropionate used as an aerosol in the treatment of asthma. Practitioner 1973;211(261):86‐90. - PubMed
Skoner 2000 {published data only}
-
- Skoner DP, Szefler SJ, Welch M, Walton‐Bowen K, Cruz‐Rivera M, Smith JA. Longitudinal growth in infants and young children treated with budesonide inhalation suspension for persistent asthma. Journal of Allergy and Clinical Immunology 2000;105(2 Pt 1):259‐68. - PubMed
Skoner 2006 {published data only}
-
- Skoner D, Maspero J, Kundu S, Lloyd M, Banerji D. Ciclesonide administered once daily has no effect on growth velocity in prepubertal children with mild persistent asthma. Journal of Allergy and Clinical Immunology 2006;117(2 Suppl 1):S11.
Skoner 2010 {published data only}
-
- Skoner DP, Gentile DA, Angelini B. Effect of therapeutic doses of mometasone furoate on cortisol levels in children with mild asthma. Allergy and Asthma Proceedings 2010;31(1):10‐19. - PubMed
Szefler 2008 {published data only}
Thompson 1998 {published data only}
-
- Thompson PJ, Davies RJ, Young WF, Grossman AB, Donnell D. Safety of hydrofluoroalkane‐134a beclomethasone dipropionate extrafine aerosol. Respiratory Medicine 1998;92(Suppl A):33‐9. - PubMed
Turpeinen 2008 {published data only}
Visser 2001 {published data only}
-
- Visser MJ, Postma DS, Arends LR, Vries TW, Duiverman EJ, Brand PL. One‐year treatment with different dosing schedules of fluticasone propionate in childhood asthma. Effects on hyperresponsiveness, lung function, and height. American Journal of Respiratory and Critical Care Medicine 2001;164(11):2073‐7. - PubMed
Visser 2001a {published data only}
-
- Visser MJ, Duiverman EJ, Postma DS, Arends LR, Brand PLP. High doses of inhaled fluticasone propionate dose‐dependently suppress adrenal cortical function in asthmatic children. European Respiratory Journal 2001;18(Suppl 33):290s.
Visser 2004 {published data only}
-
- Visser MJ, Veer E, Postma DS, Arends LR, Vries TW, Brand PLP, et al. Side‐effects of fluticasone in asthmatic children: no effects after dose reduction. European Respiratory Journal 2004;24(3):420‐5. - PubMed
Wasserman 1996 {published data only}
-
- Wasserman SI, Gross GN, Schoenwetter WF, Munk ZM, Kral KM, Schaberg A, et al. A 12‐week dose‐ranging study of fluticasone propionate powder in the treatment of asthma. Journal of Asthma 1996;33(4):265‐74. - PubMed
Wasserman 1996 b {published data only}
-
- Wasserman SI, Gross GN, Schoenwetter WF, Munk ZM, Kral KM, Schaberg A, et al. A 12‐week dose‐ranging study of fluticasone propionate powder in the treatment of asthma. Journal of Asthma 1996;33(4):265‐74. - PubMed
Waugh 2002 {published data only}
-
- Waugh J, Goa KL. Flunisolide HFA. American Journal of Respiratory Medicine 2002;1(5):369‐72; discussion 373. - PubMed
Williams 2010 {published data only}
Wolthers 1995 {published data only}
-
- Wolthers OD, Juul A, Hansen M, Muller J, Pedersen S. The insulin‐like growth factor axis and collagen turnover in asthmatic children treated with inhaled budesonide. Acta Paediatrica 1995;84:393‐7. - PubMed
Xu 2005 {published data only}
-
- Xu Z, Chen H‐Y, Zhang S‐Y, Wang X‐L, He C‐R, Qiao Y‐X. Efficacy and safety of corticosteroid inhalation in the treatment of childhood asthma. Zhongguo Dangdai Erke Zazhi 2005;7(1):47‐50.
Additional references
Adams 2011a
-
- Adams NP, Bestall JC, Lasserson TJ, Jones P, Cates CJ. Fluticasone versus placebo for chronic asthma in adults and children. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD003135.pub4] - DOI
Adams 2011b
Adams 2011c
-
- Adams NP, Bestall JB, Jones PW. Budesonide versus placebo for chronic asthma in children and adults. Cochrane Database of Systematic Reviews 1999, Issue 4. [DOI: 10.1002/14651858.CD003274] - DOI
Agertoft 2003
-
- Agertoft L, Laulund LW, Harrison LI, Pedersen S. Influence of particle size on lung deposition and pharmacokinetics of beclomethasone dipropionatein children. Pediatric Pulmonology 2003;35:192‐9. - PubMed
Agertoft 2003a
-
- Agertoft L, Pedersen S. Lung deposition and systemic availability of fluticasone Diskus and budesonide Turbuhaler in children. American Journal of Respiratory and Critical Care Medicine 2003;168:779‐82. - PubMed
Allen 1999
-
- Allen DB. Limitations of short‐term studies in predicting long‐term adverse effects of inhaled corticosteroids. Allergy 1999;54:29‐34. - PubMed
Allen 2002
-
- Allen DB. Safety of inhaled corticosteroids in children. Pediatric Pulmonology 2002;33:208‐20. - PubMed
Allen 2006
-
- Allen DB. Effects of inhaled steroids on growth, bone metabolism, and adrenal function. Advances in Pediatrics 2006;53:101‐10. - PubMed
Asher 2010
-
- Asher MI. Recent perspectives on global epidemiology of asthma in childhood. Allergologia et Immunopathologia (Madrid) 2010;38(2):83‐7. - PubMed
Barnes 2003
-
- Barnes PJ, Adcock IM. How do corticosteroids work in asthma?. Annals of Internal Medicine 2003;139:359‐70. - PubMed
Braman 2006
-
- Braman SS. The global burden of asthma. Chest 2006;130:4S‐12S. - PubMed
Brand 2001
-
- Brand PLP. Inhaled corticosteroids reduce growth. Or do they?. European Respiratory Journal 2001;17:287‐94. - PubMed
BTS 2012
-
- British Thoracic Society and Scottish Intercollegiate Guidelines Network. British Guideline on the Management of Asthma. A national clinical guideline. May 2008 (revised January 2012). http://www.google.co.uk/url?sa=t&rct=j&q=&esrc=s&source=... (accessed 20 June 2012).
CAMP Research Group 2000
-
- Szefler S, Weiss S, Tonascia J. Long‐term effects of budesonide or nedocromil in children with asthma. The New England Journal of Medecine 2000;343:1054‐63. - PubMed
CAMP Research Group 2012
Carlsen 2002
-
- Carlsen KH, Gerritsen J. Inhaled steroids in children: adrenal suppression and growth impairment. European Respiratory Journal 2002;19:985‐8. - PubMed
Chauhan 2012
Covar 2003
-
- Covar RA, Szefler SJ, Martin RJ, Sundstrom DA, Silkoff PE, Murphy J, et al. Relations between exhaled nitric oxide and measures of disease activity among children with mild‐to‐moderate asthma. Journal of Pediatrics 2003;142(5):469‐75. - PubMed
Creese 2001
-
- Creese KH, Doull IJ. Effects of inhaled corticosteroids on growth in asthmatic children. Current Allergy and Asthma Reports 2001;1(2):122‐6. - PubMed
Efthimiou 1998
-
- Efthimiou J, Barnes PJ. Effect of inhaled corticosteroids on bones and growth. European Respiratory Journal 1998;11:1167‐77. - PubMed
FDA 1998
-
- US Food, Drug Administration (FDA). FDA requires new pediatric labelling for inhaled, intranasal corticosteroids. FDA Talk Paper. November 9, 1998.
GINA 2014
-
- Global Initiative for Asthma (GINA). From the Global Strategy for Asthma Management and Prevention, Global Initiative for Asthma (GINA) 2014. http://www.ginasthma.org/local/uploads/files/GINA_Report_2014.pdf 2014.
Guilbert 2006a
-
- Guilbert TW, Morgan WJ, Zeiger RS, Mauger DT, Boehmer SJ, Szefler SJ. Long‐term inhaled corticosteroids in preschool children at high risk for asthma. The New England Journal of Medecine 2006;354:1985‐97. - PubMed
Higgins 2008
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated September 2008]. The Cochrane Collaboration. www.cochrane‐handbook.org, 2008.
ISAAC 1998
-
- ISAAC Steering Committee. Worldwide variations in the prevalence of asthma symptoms: the International Studyof Asthma and Allergies in Childhood (ISAAC). European Respiratory Journal 1998;12:315‐35. - PubMed
Juniper 1990
-
- Juniper EF, Kline PA, Vanzieleghem MA, Ramsdale EH, O'Byrne PM, Hargreave FE. Effect of long‐term treatment with an inhaled corticosteroid (budesonide) on airway hyperresponsiveness and clinical asthma in nonsteroid‐dependent asthmatics. American Review of Respiratory Disease 1990;142(4):832‐6. - PubMed
Lai 2009
-
- Lai C, Beasley R, Crane J, Foliaki S, Shah J, Weiland S. Global variation in the prevalence and severity of asthma symptoms: Phase Three of the International Study of Asthma and Allergies in Childhood (ISAAC). Thorax 2009;64(6):476‐83. - PubMed
Lougheed 2012
Martin 2002
-
- Martin RJ, Szefler SJ, Chinchilli VM, Kraft M, Dolovich M, Boushey HA, et al. Systemic effect comparisons of six inhaled corticosteroid preparations. American Journal of Respiratory and Critical Care Medicine 2002;165:1377‐83. - PubMed
Masoli 2004
-
- Masoli M, Fabian D, Holt S, Beasley R. Global Initiative for Asthma (GINA) Program. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy 2004;59(5):469‐78. - PubMed
NHLBI 2007
-
- National Heart Lung and Blood Institute. Guidelines for the diagnosis and management of asthma (EPR‐3). http://www.nhlbi.nih.gov/guidelines/asthma/ (accessed 20 June 2011).
NHLBI Expert Panel Report 2012
-
- Expert Panel Report 2007. Guidelines for the Diagnosis and Management of Asthma [National Heart, Lung and Blood Institute, National Institute of Health]. www.nhlbi.nih.gov.clinical practice guidelines (accessed 10 December 2012).
Olivieri 1997
-
- Olivieri D, Chetta A, Donno M, Bertorelli G, Casalini A, Pesci A, et al. Effect of short‐term treatment with low‐dose inhaled fluticasone propionate on airway inflammation and remodeling in mild asthma: a placebo‐controlled study. American Journal of Respiratory and Critical Care Medicine 1997;155(6):1864‐71. - PubMed
Pedersen 2001
-
- Pedersen S. Do inhaled corticosteroids inhibit growth in children?. Americian Journal of Respiratory and Critical Care Medicine 2001;164(4):521‐35. - PubMed
Price 2002a
-
- Price J, Hindmarsh P, Hughes S, Efthimiou J. Evaluating the effects of asthma therapy on childhood growth: what can be learnt from the published literature?. European Respiratory Journal 2002;19:1179‐93. - PubMed
Review Manager 5 [Computer program]
-
- Copenhagen, The Nordic Cochrane Centre: The Cochrane Collaboration. Review Manager (RevMan) Version 5.0. Copenhagen, The Nordic Cochrane Centre: The Cochrane Collaboration, 2008.
Salvatoni 2003
-
- Salvatoni A, Piantanida E, Nosetti L, Nespoli L. Inhaled corticosteroids in childhood asthma: long‐term effects on growth and adrenocortical function. Paediatric Drugs 2003;5(6):351‐61. - PubMed
Sharek 2000a
Sharek 2000b
-
- Sharek PJ, Bergman DA. The effect of inhaled steroids on the linear growth of children with asthma: a meta‐analysis. Pediatrics 2000;106:e8. - PubMed
Simons 1997
-
- Simons FER and Canadian Beclomethasone Dipropionate‐Salmeterol Xinafoate Study Group. A comparison of beclomethasone, salmeterol, and placebo in children with asthma. The New England Journal of Medicine 1997;337:1659‐65. - PubMed
Sizonenko 2002
-
- Sinonenko PC. Effects of inhaled or nasal glucocorticosteroids on adrenal function and growth. Journal of Pediatric Endocrinology and Metabolism 2002;15(1):5‐26. - PubMed
Skonner 2011
-
- Skoner DP, Meltzer EO, Milgrom H, Stryszak P, Teper A, Staudinger H. Effects of inhaled mometasone furoate on growth velocity and adrenal function: a placebo‐controlled trial in children 4‐9 years old with mild persistent asthma. The Journal of Asthma 2011;48:848‐59. - PubMed
Sobande 2008
-
- Sobande PO, Kercsmar CM. Inhaled corticosteroids in asthma management. Respiratory Care 2008;53(5):625‐33. - PubMed
Suissa 2000
-
- Suissa S, Ernst P, Benayoun S, Baltzan M, Cai B. Low‐dose inhaled corticosteroids and the prevention of death from asthma. New England Journal of Medicine 2000;343(5):332‐6. - PubMed
US FDA 2007
-
- Guidance for industry orally inhaled and intranasal corticosteroids: evaluation of the effects on growth in children, 2007. http://www.fda.gov/cder/guidance/index.htm.
Van Essen‐Zandvliet 1992
-
- Essen‐Zandvliet EE, Hughes MD, Waalkens HJ, Duiverman EJ, Pocock SJ, Kerrebijn KF. Effects of 22 months of treatment with inhaled corticosteroids and/or beta‐2‐agonists on lung function, airway responsiveness,and symptoms in children with asthma. American Review of Respiratory Disease 1992;146(3):547‐54. - PubMed
Van Rensen 1999
Witzmann 2000
-
- Witzmann KB, Fink RJ. Inhaled corticosteroids in childhood asthma: growing concerns. Drugs 2000;59(Suppl 1):9‐14. - PubMed
Wolthers 2001
-
- Wolthers O, Hansen M, Juul A, Nielsen HK, Pedersen S. Knemometry, urine cortisol excretion and measures of the insulin‐like growth factor axis and collagen turnover in the assessment of systemic activity of inhaled corticosteroids in children with persistent asthma: effects on growth. Pediatric Research 1997;41:44‐50. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

