A 3.8-V earth-abundant sodium battery electrode
- PMID: 25030272
- PMCID: PMC4109020
- DOI: 10.1038/ncomms5358
A 3.8-V earth-abundant sodium battery electrode
Abstract
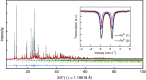
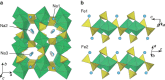
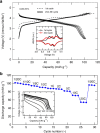
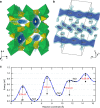
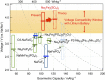
Rechargeable lithium batteries have ushered the wireless revolution over last two decades and are now matured to enable green automobiles. However, the growing concern on scarcity and large-scale applications of lithium resources have steered effort to realize sustainable sodium-ion batteries, Na and Fe being abundant and low-cost charge carrier and redox centre, respectively. However, their performance is limited owing to low operating voltage and sluggish kinetics. Here we report a hitherto-unknown material with entirely new composition and structure with the first alluaudite-type sulphate framework, Na2Fe2(SO4)3, registering the highest-ever Fe(3+)/Fe(2+) redox potential at 3.8 V (versus Na, and hence 4.1 V versus Li) along with fast rate kinetics. Rare-metal-free Na-ion rechargeable battery system compatible with the present Li-ion battery is now in realistic scope without sacrificing high energy density and high power, and paves way for discovery of new earth-abundant sustainable cathodes for large-scale batteries.
Figures
References
-
- Mizushima K., Jones P. C., Wiseman P. J. & Goodenough J. B. LixCoO2 (0<x<1): A new cathode material for batteries of high energy density. Mater. Res. Bull. 15, 783–789 (1980).
-
- Thackeray M. M., David W. I. F., Bruce P. G. & Goodenough J. B. Lithium insertion into manganese spinels. Mater. Res. Bull. 18, 461–472 (1983).
-
- Padhi A. K., Nanjundaswamy K. S. & Goodenough J. B. Phospho-olivines as positive-electrode materials for rechargeable lithium batteries. J. Electrochem. Soc. 144, 1188–1194 (1997).
-
- Tarascon J. M. Is lithium the new gold? Nat. Chem. 2, 510 (2010). - PubMed
-
- Delmas C., Braconnier J. J., Fouassier C. & Hagenmuller P. Electrochemical insertion of sodium in NaxCoO2 bronzes. Solid State Ion 3–4, 165–169 (1981).
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

