Subgroup analyses in randomised controlled trials: cohort study on trial protocols and journal publications
- PMID: 25030633
- PMCID: PMC4100616
- DOI: 10.1136/bmj.g4539
Subgroup analyses in randomised controlled trials: cohort study on trial protocols and journal publications
Erratum in
- BMJ. 2014;349:4921. Kasenda, Benjamin [added]; Schandelmaier, Stefan [added]; Sun, Xin [added]; von Elm, Erik [added]; You, John [added]; Blümle, Anette [added]; Tomonaga, Yuki (8) Saccilotto, Ramon (9) Amstutz, Alain [added]; Bengough, Theresa [added]; etc.
Abstract
Objective: To investigate the planning of subgroup analyses in protocols of randomised controlled trials and the agreement with corresponding full journal publications.
Design: Cohort of protocols of randomised controlled trial and subsequent full journal publications.
Setting: Six research ethics committees in Switzerland, Germany, and Canada.
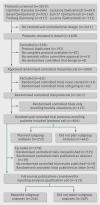
Data sources: 894 protocols of randomised controlled trial involving patients approved by participating research ethics committees between 2000 and 2003 and 515 subsequent full journal publications.
Results: Of 894 protocols of randomised controlled trials, 252 (28.2%) included one or more planned subgroup analyses. Of those, 17 (6.7%) provided a clear hypothesis for at least one subgroup analysis, 10 (4.0%) anticipated the direction of a subgroup effect, and 87 (34.5%) planned a statistical test for interaction. Industry sponsored trials more often planned subgroup analyses compared with investigator sponsored trials (195/551 (35.4%) v 57/343 (16.6%), P<0.001). Of 515 identified journal publications, 246 (47.8%) reported at least one subgroup analysis. In 81 (32.9%) of the 246 publications reporting subgroup analyses, authors stated that subgroup analyses were prespecified, but this was not supported by 28 (34.6%) corresponding protocols. In 86 publications, authors claimed a subgroup effect, but only 36 (41.9%) corresponding protocols reported a planned subgroup analysis.
Conclusions: Subgroup analyses are insufficiently described in the protocols of randomised controlled trials submitted to research ethics committees, and investigators rarely specify the anticipated direction of subgroup effects. More than one third of statements in publications of randomised controlled trials about subgroup prespecification had no documentation in the corresponding protocols. Definitive judgments regarding credibility of claimed subgroup effects are not possible without access to protocols and analysis plans of randomised controlled trials.
© The DISCO study group 2014.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at
Figures
References
-
- Hernandez AV, Boersma E, Murray GD, Habbema JD, Steyerberg EW. Subgroup analyses in therapeutic cardiovascular clinical trials: are most of them misleading? Am Heart J 2006;151:257-64. - PubMed
-
- Wang R, Lagakos SW, Ware JH, Hunter DJ, Drazen JM. Statistics in medicine--reporting of subgroup analyses in clinical trials. N Engl J Med 2007;357:2189-94. - PubMed
-
- Assmann SF, Pocock SJ, Enos LE, Kasten LE. Subgroup analysis and other (mis)uses of baseline data in clinical trials. Lancet 2000;355:1064-9. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources