Endocannabinoids modulate cortical development by configuring Slit2/Robo1 signalling
- PMID: 25030704
- PMCID: PMC4110686
- DOI: 10.1038/ncomms5421
Endocannabinoids modulate cortical development by configuring Slit2/Robo1 signalling
Abstract
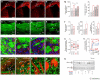
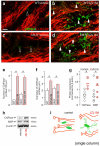
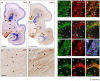
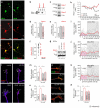
Local environmental cues are indispensable for axonal growth and guidance during brain circuit formation. Here, we combine genetic and pharmacological tools, as well as systems neuroanatomy in human fetuses and mouse models, to study the role of endocannabinoid and Slit/Robo signalling in axonal growth. We show that excess 2-arachidonoylglycerol, an endocannabinoid affecting directional axonal growth, triggers corpus callosum enlargement due to the errant CB1 cannabinoid receptor-containing corticofugal axon spreading. This phenotype mechanistically relies on the premature differentiation and end-feet proliferation of CB2R-expressing oligodendrocytes. We further show the dependence of both axonal Robo1 positioning and oligodendroglial Slit2 production on cell-type-specific cannabinoid receptor activation. Accordingly, Robo1 and/or Slit2 manipulation limits endocannabinoid modulation of axon guidance. We conclude that endocannabinoids can configure focal Slit2/Robo1 signalling to modulate directional axonal growth, which may provide a basis for understanding impaired brain wiring associated with metabolic deficits and prenatal drug exposure.
Figures



References
-
- Brose K, et al. Slit proteins bind Robo receptors and have an evolutionarily conserved role in repulsive axon guidance. Cell. 1999;96:795–806. - PubMed
-
- Hohenester E. Structural insight into Slit-Robo signalling. Biochem. Soc. Trans. 2008;36:251–256. - PubMed
-
- Andrews W, et al. Robo1 regulates the development of major axon tracts and interneuron migration in the forebrain. Development. 2006;133:2243–2252. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

