Expression profile analysis of hippocampal CA1 pyramidal neurons in aged Ts65Dn mice, a model of Down syndrome (DS) and Alzheimer's disease (AD)
- PMID: 25031177
- PMCID: PMC4297601
- DOI: 10.1007/s00429-014-0839-0
Expression profile analysis of hippocampal CA1 pyramidal neurons in aged Ts65Dn mice, a model of Down syndrome (DS) and Alzheimer's disease (AD)
Abstract
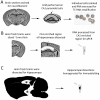
Down syndrome (DS) is caused by the triplication of human chromosome 21 (HSA21) and is the most common genetic cause of intellectual disability, with individuals having deficits in cognitive function including hippocampal learning and memory and neurodegeneration of cholinergic basal forebrain neurons, a pathological hallmark of Alzheimer's disease (AD). To date, the molecular underpinnings driving this pathology have not been elucidated. The Ts65Dn mouse is a segmental trisomy model of DS and like DS/AD pathology, displays age-related cognitive dysfunction and basal forebrain cholinergic neuron (BFCN) degeneration. To determine molecular and cellular changes important for elucidating mechanisms of neurodegeneration in DS/AD pathology, expression profiling studies were performed. Molecular fingerprinting of homogeneous populations of Cornu Ammonis 1 (CA1) pyramidal neurons was performed via laser capture microdissection followed by Terminal Continuation RNA amplification combined with custom-designed microarray analysis and subsequent validation of individual transcripts by qPCR and protein analysis via immunoblotting. Significant alterations were observed within CA1 pyramidal neurons of aged Ts65Dn mice compared to normal disomic (2N) littermates, notably in excitatory and inhibitory neurotransmission receptor families and neurotrophins, including brain-derived neurotrophic factor as well as several cognate neurotrophin receptors. Examining gene and protein expression levels after the onset of BFCN degeneration elucidated transcriptional and translational changes in neurons within a vulnerable circuit that may cause the AD-like pathology seen in DS as these individuals age, and provide rational targets for therapeutic interventions.
Figures


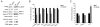

References
-
- Akeson E, Lambert J. Ts65Dn-localization of the translocation breakpoint and trisomic gene content in a mouse model for Down syndrome. Cytogenet Cell Genet. 2001;276:270–276. - PubMed
-
- Applied Biosystems Guide to Performing Relative Quantitation of Gene Expression Using Real-Time Quantitative PCR. Gene Expr. 2008;2009:1–60.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

