SAMBA HIV semiquantitative test, a new point-of-care viral-load-monitoring assay for resource-limited settings
- PMID: 25031444
- PMCID: PMC4313165
- DOI: 10.1128/JCM.00593-14
SAMBA HIV semiquantitative test, a new point-of-care viral-load-monitoring assay for resource-limited settings
Abstract
Routine viral-load (VL) testing of HIV-infected individuals on antiretroviral therapy (ART) is used to monitor treatment efficacy. However, due to logistical challenges, implementation of VL has been difficult in resource-limited settings. The aim of this study was to evaluate the performance of the SAMBA semi-Q (simple amplification-based assay semiquantitative test for HIV-1) in London, Malawi, and Uganda. The SAMBA semi-Q can distinguish between patients with VLs above and below 1,000 copies/ml. The SAMBA semi-Q was validated with diluted clinical samples and blinded plasma samples collected from HIV-1-positive individuals. SAMBA semi-Q results were compared with results from the Roche COBAS AmpliPrep/COBAS TaqMan HIV-1 test, v2.0. Testing of 96 2- to 10-fold dilutions of four samples containing HIV-1 subtype C as well as 488 samples from patients in the United Kingdom, Malawi, and Uganda yielded an overall accuracy for the SAMBA semi-Q of 99% (95% confidence interval [CI], 93.8 to 99.9%) and 96.9% (95% CI 94.9 to 98.3%), respectively, compared to to the Roche test. Analysis of VL data from patients in Malawi and Uganda showed that the SAMBA cutoff of 1,000 copies/ml appropriately distinguished treated from untreated individuals. Furthermore, analysis of the viral loads of 232 patients on ART in Malawi and Uganda revealed similar patterns for virological control, defined as either <1,000 copies/ml (SAMBA cutoff) or <5,000 copies/ml (WHO 2010 criterion; WHO, Antiretroviral Therapy for HIV Infection in Adults and Adolescents: Recommendations for a Public Health Approach, 2010). This study suggests that the SAMBA semi-Q has adequate concurrency with the gold standard measurements for viral load. This test can allow VL monitoring of patients on ART at the point of care in resource-limited settings.
Copyright © 2014 Ritchie et al.
Figures

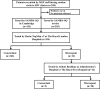
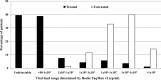
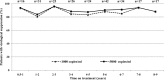
References
-
- WHO. 2013. HIV/AIDS: data and statistics. World Health Organization, Geneva, Switzerland.
-
- Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, Hakim JG, Kumwenda J, Grinsztejn B, Pilotto JH, Godbole SV, Mehendale S, Chariyalertsak S, Santos BR, Mayer KH, Hoffman IF, Eshleman SH, Piwowar-Manning E, Wang L, Makhema J, Mills LA, de Bruyn G, Sanne I, Eron J, Gallant J, Havlir D, Swindells S, Ribaudo H, Elharrar V, Burns D, Taha TE, Nielsen-Saines K, Celentano D, Essex M, Fleming TR, HPTN 052 Study Team 2011. Prevention of HIV-1 infection with early antiretroviral therapy. N. Engl. J. Med. 365:493–505. 10.1056/NEJMoa1105243. - DOI - PMC - PubMed
-
- United Nations. 2011. Political declaration on HIV/AIDS: Intensifying our efforts to eliminate HIV/AIDS. United Nations.
-
- Rawizza HE, Chaplin B, Meloni ST, Eisen G, Rao T, Sankale JL, Dieng-Sarr A, Agbaji O, Onwujekwe DI, Gashau W, Nkado R, Ekong E, Okonkwo P, Murphy RL, Kanki PJ. 2011. Immunologic criteria are poor predictors of virologic outcome: implications for HIV treatment monitoring in resource-limited settings. Clin. Infect. Dis. 53:1283–1290. 10.1093/cid/cir729. - DOI - PMC - PubMed
-
- Fox MP, Cutsem GV, Giddy J, Maskew M, Keiser O, Prozesky H, Wood R, Hernán MA, Sterne JA, Egger M, Boulle A, IeDEA-SA Collaboration 2012. Rates and predictors of failure of first-line antiretroviral therapy and switch to second-line ART in South Africa. J. Acquir. Immune Defic. Syndr. 60:428–437. 10.1097/QAI.0b013e3182557785. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

