Phage therapy: eco-physiological pharmacology
- PMID: 25031881
- PMCID: PMC4054669
- DOI: 10.1155/2014/581639
Phage therapy: eco-physiological pharmacology
Abstract
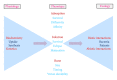
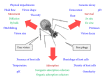
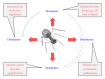
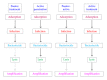
Bacterial virus use as antibacterial agents, in the guise of what is commonly known as phage therapy, is an inherently physiological, ecological, and also pharmacological process. Physiologically we can consider metabolic properties of phage infections of bacteria and variation in those properties as a function of preexisting bacterial states. In addition, there are patient responses to pathogenesis, patient responses to phage infections of pathogens, and also patient responses to phage virions alone. Ecologically, we can consider phage propagation, densities, distribution (within bodies), impact on body-associated microbiota (as ecological communities), and modification of the functioning of body "ecosystems" more generally. These ecological and physiological components in many ways represent different perspectives on otherwise equivalent phenomena. Comparable to drugs, one also can view phages during phage therapy in pharmacological terms. The relatively unique status of phages within the context of phage therapy as essentially replicating antimicrobials can therefore result in a confluence of perspectives, many of which can be useful towards gaining a better mechanistic appreciation of phage therapy, as I consider here. Pharmacology more generally may be viewed as a discipline that lies at an interface between organism-associated phenomena, as considered by physiology, and environmental interactions as considered by ecology.
Figures








References
-
- Tracy CR, Turner JS. What is physiological ecology? Bulletin of the Ecological Society of America. 1982;63:340–347.
-
- Brüssow H, Kutter E. Phage ecology. In: Kutter E, Sulakvelidze A, editors. Bacteriophages: Biology and Applications. Boca Raton, Fla, USA: CRC Press; 2005. pp. 129–164.
-
- Lee HC, Lai K, Lorenc MT, Imelfort M, Duran C, Edwards D. Bioinformatics tools and databases for analysis of next-generation sequence data. Briefings in Functional Genomics. 2012;11(1):12–24. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

