Pneumococcal vaccines: understanding centers for disease control and prevention recommendations
- PMID: 25032872
- PMCID: PMC4213993
- DOI: 10.1513/AnnalsATS.201401-042CME
Pneumococcal vaccines: understanding centers for disease control and prevention recommendations
Abstract
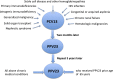
Streptococcus pneumoniae infection is a common and serious health problem that is best prevented by the pneumococcal vaccine. The first vaccine approved by the U.S. Federal Drug Administration in 1977 contained 14 polysaccharide antigens. An improved vaccine introduced in 1983 included 23 polysaccharide antigens. Both vaccines were effective for immunocompetent adults; however, young children and immunocompromised adults remained susceptible. A pediatric vaccine was developed consisting of the capsular antigens of seven pneumococcal serotypes commonly found in children. The antigens in this preparation are covalently conjugated to diphtheria protein to make them more antigenic. The conjugate vaccine was expanded to include 13 serotypes by 2010. Although more immunogenic, the conjugate vaccine has fewer serotypes than the older 23-valent vaccine. The U.S. Centers for Disease Control and Prevention recommend that children at risk for pneumococcal pneumonia as defined by the presence of chronic disease should receive the 13-valent conjugated vaccine. Adults at risk for pneumococcal pneumonia, which includes those over 65 years of age and those who have a chronic disease, should receive the 23-polysaccharide vaccine. Immunosuppressed patients of any age should receive both vaccines. Adults should be revaccinated once at age 65 years or older with the 23-polysaccharide vaccine provided that at least 5 years have elapsed since the previous vaccination.
Keywords: PCV13; PPV23; adults; pneumococcal vaccine; vaccination.
Figures
References
-
- World Health Organization Pneumococcal vaccines Geneva, Switzerland: World Health Organization; 2013. Available from: http://www.who.int/biologicals/areas/vaccines/pneumo/en/
-
- Black RE, Cousens S, Johnson HL, Lawn JE, Rudan I, Bassani DG, Jha P, Campbell H, Walker CF, Cibulskis R, et al. Child Health Epidemiology Reference Group of WHO and UNICEF. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010;375:1969–1987. - PubMed
-
- Yu H, Rubin J, Dunning S, Li S, Sato R. Clinical and economic burden of community-acquired pneumonia in the Medicare fee-for-service population. J Am Geriatr Soc. 2012;60:2137–2143. - PubMed
-
- Aliberti S, Kaye KS. The changing microbiologic epidemiology of community-acquired pneumonia. Postgrad Med. 2013;125:31–42. - PubMed
-
- Robinson KA, Baughman W, Rothrock G, Barrett NL, Pass M, Lexau C, Damaske B, Stefonek K, Barnes B, Patterson J, et al. Active Bacterial Core Surveillance (ABCs)/Emerging Infections Program Network. Epidemiology of invasive Streptococcus pneumoniae infections in the United States, 1995–1998: opportunities for prevention in the conjugate vaccine era. JAMA. 2001;285:1729–1735. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical