A novel class of bis- and tris-chelate diam(m)inebis(dicarboxylato)platinum(IV) complexes as potential anticancer prodrugs
- PMID: 25032896
- PMCID: PMC4351917
- DOI: 10.1021/jm500791c
A novel class of bis- and tris-chelate diam(m)inebis(dicarboxylato)platinum(IV) complexes as potential anticancer prodrugs
Abstract
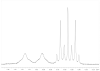
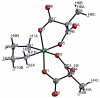
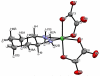
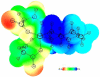
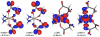
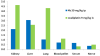
A novel class of platinum(IV) complexes of the type [Pt(Am)(R(COO)2)2], where Am is a chelating diamine or two monodentate am(m)ine ligands and R(COO)2 is a chelating dicarboxylato moiety, was synthesized. For this purpose, the reaction between the corresponding tetrahydroxidoplatinum(IV) precursors and various dicarboxylic acids, such as oxalic, malonic, 3-methylmalonic, and cyclobutanedicarboxylic acid, was utilized. All new compounds were characterized in detail, using 1D and 2D NMR techniques, ESI-MS, FTIR spectroscopy, elemental analysis, TGA, and X-ray diffraction. Their in vitro cytotoxicity was determined in a panel of human tumor cell lines (CH1, SW480 and A549) by means of the MTT colorimetric assay. Furthermore, the lipophilicity and redox properties of the novel complexes were evaluated in order to better understand their pharmacological behavior. The most promising drug candidate, 4b (Pt(DACH)(mal)2), demonstrated low in vivo toxicity but profound anticancer activity against both the L1210 leukemia and CT-26 colon carcinoma models.
Figures




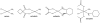
References
-
- Wheate NJ, Walker S, Craig GE, Oun R. The status of platinum anticancer drugs in the clinic and in clinical trials. Dalton Trans. 2010;39:8113–8127. - PubMed
-
- Einhorn LH. Cisplatin and the revolution of treatment of germ cell tumors; 11th International Symposium on Platinum Coordination Compounds in Cancer Chemotherapy; Verona, Italy. October 11–14, 2012.
-
- Galanski M, Jakupec MA, Keppler BK. Update of the preclinical situation of anticancer platinum complexes: novel design strategies and innovative analytical approaches. Curr. Med. Chem. 2005;12:2075–2094. - PubMed
-
- Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat. Rev. Cancer. 2007;7:573–584. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

