Decay-accelerating factor 1 deficiency exacerbates leptospiral-induced murine chronic nephritis and renal fibrosis
- PMID: 25032961
- PMCID: PMC4102560
- DOI: 10.1371/journal.pone.0102860
Decay-accelerating factor 1 deficiency exacerbates leptospiral-induced murine chronic nephritis and renal fibrosis
Abstract
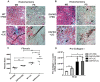
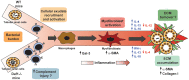
Leptospirosis is a global zoonosis caused by pathogenic Leptospira, which can colonize the proximal renal tubules and persist for long periods in the kidneys of infected hosts. Here, we characterized the infection of C57BL/6J wild-type and Daf1-/- mice, which have an enhanced host response, with a virulent Leptospira interrogans strain at 14 days post-infection, its persistence in the kidney, and its link to kidney fibrosis at 90 days post-infection. We found that Leptospira interrogans can induce acute moderate nephritis in wild-type mice and is able to persist in some animals, inducing fibrosis in the absence of mortality. In contrast, Daf1-/- mice showed acute mortality, with a higher bacterial burden. At the chronic stage, Daf1-/- mice showed greater inflammation and fibrosis than at 14 days post-infection and higher levels at all times than the wild-type counterpart. Compared with uninfected mice, infected wild-type mice showed higher levels of IL-4, IL-10 and IL-13, with similar levels of α-smooth muscle actin, galectin-3, TGF-β1, IL-17, IFN-γ, and lower IL-12 levels at 90 days post-infection. In contrast, fibrosis in Daf1-/- mice was accompanied by high expression of α-smooth muscle actin, galectin-3, IL-10, IL-13, and IFN-γ, similar levels of TGF-β1, IL-12, and IL-17 and lower IL-4 levels. This study demonstrates the link between Leptospira-induced murine chronic nephritis with renal fibrosis and shows a protective role of Daf1.
Conflict of interest statement
Figures





References
-
- Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, et al. (2007) Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047. - PubMed
-
- Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, et al. (2013) Chronic kidney disease: global dimension and perspectives. Lancet 382: 260–272. - PubMed
-
- Faine S, Adler B, Bolin C, Perolat P (1999) Leptospira and Leptospirosis. Melbourne, Australia MediSci.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

