Oroya fever and verruga peruana: bartonelloses unique to South America
- PMID: 25032975
- PMCID: PMC4102455
- DOI: 10.1371/journal.pntd.0002919
Oroya fever and verruga peruana: bartonelloses unique to South America
Abstract
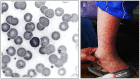
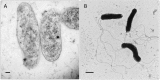
Bartonella bacilliformis is the bacterial agent of Carrión's disease and is presumed to be transmitted between humans by phlebotomine sand flies. Carrión's disease is endemic to high-altitude valleys of the South American Andes, and the first reported outbreak (1871) resulted in over 4,000 casualties. Since then, numerous outbreaks have been documented in endemic regions, and over the last two decades, outbreaks have occurred at atypical elevations, strongly suggesting that the area of endemicity is expanding. Approximately 1.7 million South Americans are estimated to be at risk in an area covering roughly 145,000 km2 of Ecuador, Colombia, and Peru. Although disease manifestations vary, two disparate syndromes can occur independently or sequentially. The first, Oroya fever, occurs approximately 60 days following the bite of an infected sand fly, in which infection of nearly all erythrocytes results in an acute hemolytic anemia with attendant symptoms of fever, jaundice, and myalgia. This phase of Carrión's disease often includes secondary infections and is fatal in up to 88% of patients without antimicrobial intervention. The second syndrome, referred to as verruga peruana, describes the endothelial cell-derived, blood-filled tumors that develop on the surface of the skin. Verrugae are rarely fatal, but can bleed and scar the patient. Moreover, these persistently infected humans provide a reservoir for infecting sand flies and thus maintaining B. bacilliformis in nature. Here, we discuss the current state of knowledge regarding this life-threatening, neglected bacterial pathogen and review its host-cell parasitism, molecular pathogenesis, phylogeny, sand fly vectors, diagnostics, and prospects for control.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


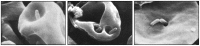
References
-
- Hertig M (1942) Phlebotomus and Carrión's Disease. Am J Trop Med Hyg S1-22: 2–81.
-
- Weinman D (1965) The bartonella group. In: Dubos RJ, Hirsch JG, editors. Bacterial and Mycotic Infections of Man. Philadelphia: Lippincott. pp. 775–785.
-
- Birtles RJ, Canales J, Ventosilla P, Alvarez E, Guerra H, et al. (1999) Survey of Bartonella species infecting intradomicillary animals in the Huayllacallán Valley, Ancash, Peru, a region endemic for human bartonellosis. Am J Trop Med Hyg 60: 799–805. - PubMed
-
- Maguiña C, Gotuzzo E (2000) Bartonellosis. New and old. Infect Dis Clin North Am 14: 1–22. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

