Microsatellite interruptions stabilize primate genomes and exist as population-specific single nucleotide polymorphisms within individual human genomes
- PMID: 25033203
- PMCID: PMC4102424
- DOI: 10.1371/journal.pgen.1004498
Microsatellite interruptions stabilize primate genomes and exist as population-specific single nucleotide polymorphisms within individual human genomes
Abstract
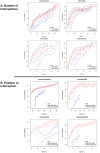
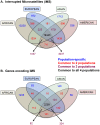
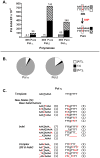
Interruptions of microsatellite sequences impact genome evolution and can alter disease manifestation. However, human polymorphism levels at interrupted microsatellites (iMSs) are not known at a genome-wide scale, and the pathways for gaining interruptions are poorly understood. Using the 1000 Genomes Phase-1 variant call set, we interrogated mono-, di-, tri-, and tetranucleotide repeats up to 10 units in length. We detected ∼26,000-40,000 iMSs within each of four human population groups (African, European, East Asian, and American). We identified population-specific iMSs within exonic regions, and discovered that known disease-associated iMSs contain alleles present at differing frequencies among the populations. By analyzing longer microsatellites in primate genomes, we demonstrate that single interruptions result in a genome-wide average two- to six-fold reduction in microsatellite mutability, as compared with perfect microsatellites. Centrally located interruptions lowered mutability dramatically, by two to three orders of magnitude. Using a biochemical approach, we tested directly whether the mutability of a specific iMS is lower because of decreased DNA polymerase strand slippage errors. Modeling the adenomatous polyposis coli tumor suppressor gene sequence, we observed that a single base substitution interruption reduced strand slippage error rates five- to 50-fold, relative to a perfect repeat, during synthesis by DNA polymerases α, β, or η. Computationally, we demonstrate that iMSs arise primarily by base substitution mutations within individual human genomes. Our biochemical survey of human DNA polymerase α, β, δ, κ, and η error rates within certain microsatellites suggests that interruptions are created most frequently by low fidelity polymerases. Our combined computational and biochemical results demonstrate that iMSs are abundant in human genomes and are sources of population-specific genetic variation that may affect genome stability. The genome-wide identification of iMSs in human populations presented here has important implications for current models describing the impact of microsatellite polymorphisms on gene expression.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, et al. (2001) Initial sequencing and analysis of the human genome. Nature 409: 860–921. - PubMed
-
- Ellegren H (2004) Microsatellites: simple sequences with complex evolution. Nat Rev Genet 5: 435–445. - PubMed
-
- Pearson CE, Nichol Edamura K, Cleary JD (2005) Repeat instability: mechanisms of dynamic mutations. Nat Rev Genet 6: 729–742. - PubMed
-
- Gemayel R, Vinces MD, Legendre M, Verstrepen KJ (2010) Variable tandem repeats accelerate evolution of coding and regulatory sequences. Annu Rev Genet 44: 445–477. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

