Differential activation of intracellular versus plasmalemmal CB2 cannabinoid receptors
- PMID: 25033246
- PMCID: PMC4144709
- DOI: 10.1021/bi500632a
Differential activation of intracellular versus plasmalemmal CB2 cannabinoid receptors
Abstract
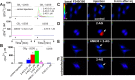
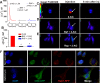
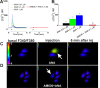
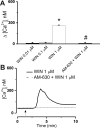
The therapeutic and psychoactive properties of cannabinoids have long been recognized. The type 2 receptor for cannabinoids (CB2) has emerged as an important therapeutic target in several pathologies, as it mediates beneficial effects of cannabinoids while having little if any psychotropic activity. Difficulties associated with the development of CB2-based therapeutic agents have been related to its intricate pharmacology, including the species specificity and functional selectivity of the CB2-initiated responses. We postulated that a plasmalemmal or subcellular location of the receptor may contribute to the differential signaling pathways initiated by its activation. To differentiate between these two, we used extracellular and intracellular administration of CB2 ligands and concurrent calcium imaging in CB2-expressing U2OS cells. We found that extracellular administration of anandamide was ineffective, whereas 2-arachidonoyl glycerol (2-AG) and WIN55,212-2 triggered delayed, CB2-dependent Ca(2+) responses that were Gq protein-mediated. When microinjected, all agonists elicited fast, transient, and dose-dependent elevations in intracellular Ca(2+) concentration upon activation of Gq-coupled CB2 receptors. The CB2 dependency was confirmed by the sensitivity to AM630, a selective CB2 antagonist, and by the unresponsiveness of untransfected U2OS cells to 2-AG, anandamide, or WIN55,212-2. Moreover, we provide functional and morphological evidence that CB2 receptors are localized at the endolysosomes, while their activation releases Ca(2+) from inositol 1,4,5-trisphosphate-sensitive- and acidic-like Ca(2+) stores. Our results support the functionality of intracellular CB2 receptors and their ability to couple to Gq and elicit Ca(2+) signaling. These findings add further complexity to CB2 receptor pharmacology and argue for careful consideration of receptor localization in the development of CB2-based therapeutic agents.
Figures




References
-
- Pertwee R. G.; Howlett A. C.; Abood M. E.; Alexander S. P.; Di Marzo V.; Elphick M. R.; Greasley P. J.; Hansen H. S.; Kunos G.; Mackie K.; Mechoulam R.; Ross R. A. (2010) International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: Beyond CB1 and CB2. Pharmacol. Rev. 62, 588–5631. - PMC - PubMed
-
- Riether D. (2012) Selective cannabinoid receptor 2 modulators: A patent review 2009–present. Expert Opin. Ther. Pat. 22, 495–510. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

