Divergent functions of Toll-like receptors during bacterial lung infections
- PMID: 25033332
- PMCID: PMC4299612
- DOI: 10.1164/rccm.201406-1101PP
Divergent functions of Toll-like receptors during bacterial lung infections
Abstract
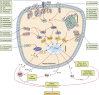
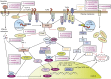
Lower respiratory tract infections caused by bacteria are a major cause of death in humans irrespective of sex, race, or geography. Indeed, accumulated data indicate greater mortality and morbidity due to these infections than cancer, malaria, or HIV infection. Successful recognition of, followed by an appropriate response to, bacterial pathogens in the lungs is crucial for effective pulmonary host defense. Although the early recruitment and activation of neutrophils in the lungs is key in the response against invading microbial pathogens, other sentinels, such as alveolar macrophages, epithelial cells, dendritic cells, and CD4(+) T cells, also contribute to the elimination of the bacterial burden. Pattern recognition receptors, such as Toll-like receptors (TLRs) and nucleotide-binding oligomerization domain-like receptors, are important for recognizing and responding to microbes during pulmonary infections. However, bacterial pathogens have acquired crafty evasive strategies to circumvent the pattern recognition receptor response and thus establish infection. Increased understanding of the function of TLRs and evasive mechanisms used by pathogens during pulmonary infection will deepen our knowledge of immunopathogenesis and is crucial for developing effective therapeutic and/or prophylactic measures. This review summarizes current knowledge of the multiple roles of TLRs in bacterial lung infections and highlights the mechanisms used by pathogens to modulate or interfere with TLR signaling in the lungs.
Keywords: Toll-like receptor; bacterial infection; evasion of Toll-like receptor signaling; lung.
Figures
References
-
- The United Nations Children’s Fund (UNICEF)/World Health OrganizationWHO), Geneva. Pneumonia: the forgotten killer of children. 2006 [accessed 2014 May 5]. Available from: http://whqlibdoc.who.int/publications/2006/9280640489_eng.pdf
-
- Opitz B, van Laak V, Eitel J, Suttorp N. Innate immune recognition in infectious and noninfectious diseases of the lung. Am J Respir Crit Care Med. 2010;181:1294–1309. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

