Molecular genetics of Müllerian duct formation, regression and differentiation
- PMID: 25033758
- PMCID: PMC4378544
- DOI: 10.1159/000364935
Molecular genetics of Müllerian duct formation, regression and differentiation
Abstract
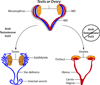
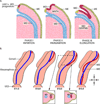
The Müllerian duct (MD) forms the female reproductive tract (FRT) consisting of the oviducts, uterus, cervix, and upper vagina. FRT function is vital to fertility, providing the site of fertilization, embryo implantation and fetal development. Developmental defects in the formation and diseases of the FRT, including cancer and endometriosis, are prevalent in humans and can result in infertility and death. Furthermore, because the MDs are initially formed regardless of genotypic sex, mesenchymal to epithelial signaling is required in males to mediate MD regression and prevents the development of MD-derived organs. In males, defects in MD regression result in the retention of FRT organs and have been described in several human syndromes. Although to date not reported in humans, ectopic activation of MD regression signaling components in females can result in aplasia of the FRT. Clearly, MD development is important to human health; however, the molecular mechanisms remain largely undetermined. Molecular genetics studies of human diseases and mouse models have provided new insights into molecular signaling during MD development, regression and differentiation. This review will provide an overview of MD development and important genes and signaling mechanisms involved.
Figures


References
-
- Allard S, Adin P, Gouedard L, di Clemente N, Josso N, Orgebin-Crist MC, Picard JY, Xavier F. Molecular mechanisms of hormone-mediated Mullerian duct regression: involvement of beta-catenin. Development. 2000;127:3349–3360. - PubMed
-
- Arango NA, Kobayashi A, Wang Y, Jamin SP, Lee HH, Orvis GD, Behringer RR. A mesenchymal perspective of Mullerian duct differentiation and regression in Amhr2-lacZ mice. Mol Reprod Dev. 2008;75:1154–1162. - PubMed
-
- Arango NA, Lovell-Badge R, Behringer RR. Targeted mutagenesis of the endogenous mouse Mis gene promoter: in vivo definition of genetic pathways of vertebrate sexual development. Cell. 1999;99:409–419. - PubMed
-
- Arango NA, Szotek PP, Manganaro TF, Oliva E, Donahoe PK, Teixeira J. Conditional deletion of beta-catenin in the mesenchyme of the developing mouse uterus results in a switch to adipogenesis in the myometrium. Dev Biol. 2005;288:276–283. - PubMed
-
- Austin HB. DiI analysis of cell migration during mullerian duct regression. Dev Biol. 1995;169:29–36. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

