Biomarkers and surrogate endpoints in glaucoma clinical trials
- PMID: 25034049
- PMCID: PMC4523640
- DOI: 10.1136/bjophthalmol-2014-305550
Biomarkers and surrogate endpoints in glaucoma clinical trials
Abstract
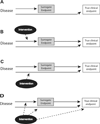
Surrogate endpoints are often used as replacements for true clinically relevant endpoints in several areas of medicine, as they enable faster and less expensive clinical trials. However, without proper validation, the use of surrogates may lead to incorrect conclusions about the efficacy and safety of treatments. This article reviews the general requirements for validating surrogate endpoints and provides a critical assessment of the use of intraocular pressure (IOP), visual fields, and structural measurements of the optic nerve as surrogate endpoints in glaucoma clinical trials. A valid surrogate endpoint must be able to predict the clinically relevant endpoint and fully capture the effect of an intervention on that endpoint. Despite its widespread use in clinical trials, no proper validation of IOP as a surrogate endpoint has ever been conducted for any class of IOP-lowering treatments. Evidence has accumulated with regard to the role of imaging measurements of optic nerve damage as surrogate endpoints in glaucoma. These measurements are predictive of functional losses in the disease and may explain, at least in part, treatment effects on clinically relevant endpoints. The use of composite endpoints in glaucoma trials may overcome weaknesses of the use of structural or functional endpoints in isolation. Unless research is dedicated to fully develop and validate suitable endpoints that can be used in glaucoma clinical trials, we run the risk of inappropriate judgments about the value of new therapies.
Keywords: Clinical Trial; Glaucoma; Imaging; Intraocular Pressure.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Figures

References
-
- Lesko LJ, Atkinson AJ., Jr Use of biomarkers and surrogate endpoints in drug development and regulatory decision making: criteria, validation, strategies. Annu Rev Pharmacol Toxicol. 2001;41:347–366. - PubMed
-
- Biomarkers Definitions Working G. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69:89–95. - PubMed
-
- Boissel JP, Collet JP, Moleur P, et al. Surrogate endpoints: a basis for a rational approach. Eur J Clin Pharmacol. 1992;43:235–244. - PubMed
-
- Prentice RL. Surrogate endpoints in clinical trials: definition and operational criteria. Stat Med. 1989;8:431–440. - PubMed
-
- Gobburu JV. Biomarkers in clinical drug development. Clin Pharmacol Ther. 2009;86:26–27. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
