Use of influenza antiviral agents by ambulatory care clinicians during the 2012-2013 influenza season
- PMID: 25034419
- PMCID: PMC4200045
- DOI: 10.1093/cid/ciu422
Use of influenza antiviral agents by ambulatory care clinicians during the 2012-2013 influenza season
Abstract
Background: Early antiviral treatment (≤2 days since illness onset) of influenza reduces the probability of influenza-associated complications. Early empiric antiviral treatment is recommended for those with suspected influenza at higher risk for influenza complications regardless of their illness severity. We describe antiviral receipt among outpatients with acute respiratory illness (ARI) and antibiotic receipt among patients with influenza.
Methods: We analyzed data from 5 sites in the US Influenza Vaccine Effectiveness Network Study during the 2012-2013 influenza season. Subjects were outpatients aged ≥6 months with ARI defined by cough of ≤7 days' duration; all were tested for influenza by polymerase chain reaction (PCR). Medical history and prescription information were collected by medical and pharmacy records. Four sites collected prescribing data on 3 common antibiotics (amoxicillin-clavulanate, amoxicillin, and azithromycin).
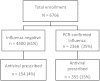
Results: Of 6766 enrolled ARI patients, 509 (7.5%) received an antiviral prescription. Overall, 2366 (35%) had PCR-confirmed influenza; 355 (15%) of those received an antiviral prescription. Among 1021 ARI patients at high risk for influenza complications (eg, aged <2 years or ≥65 years or with ≥1 chronic medical condition) presenting to care ≤2 days from symptom onset, 195 (19%) were prescribed an antiviral medication. Among participants with PCR-confirmed influenza and antibiotic data, 540 of 1825 (30%) were prescribed 1 of 3 antibiotics; 297 of 1825 (16%) were prescribed antiviral medications.
Conclusions: Antiviral treatment was prescribed infrequently among outpatients with influenza for whom therapy would be most beneficial; in contrast, antibiotic prescribing was more frequent. Continued efforts to educate clinicians on appropriate antibiotic and antiviral use are essential to improve healthcare quality.
Keywords: ambulatory care; antiviral treatment; influenza; neuraminidase inhibitors.
Published by Oxford University Press on behalf of the Infectious Diseases Society of America 2014. This work is written by (a) US Government employee(s) and is in the public domain in the US.
Figures


Comment in
-
Editorial commentary: failing our patients by suboptimally treating influenza infections.Clin Infect Dis. 2014 Sep 15;59(6):783-6. doi: 10.1093/cid/ciu425. Epub 2014 Jul 16. Clin Infect Dis. 2014. PMID: 25034418 No abstract available.
References
-
- Ebell MH, Call M, Shinholser J. Effectiveness of oseltamivir in adults: a meta-analysis of published and unpublished clinical trials. Fam Pract. 2013;30:125–33. - PubMed
-
- Torres JP, O'Ryan M, Herve B, et al. Impact of the novel influenza A (H1N1) during the 2009 autumn-winter season in a large hospital setting in Santiago, Chile. Clin Infect Dis. 2010;50:860–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

