Quantitative autofluorescence and cell density maps of the human retinal pigment epithelium
- PMID: 25034602
- PMCID: PMC4123894
- DOI: 10.1167/iovs.14-14802
Quantitative autofluorescence and cell density maps of the human retinal pigment epithelium
Abstract
Purpose: Lipofuscin (LF) accumulation within RPE cells is considered pathogenic in AMD. To test whether LF contributes to RPE cell loss in aging and to provide a cellular basis for fundus autofluorescence (AF) we created maps of human RPE cell number and histologic AF.
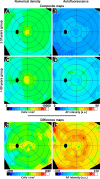
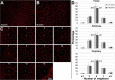
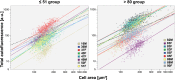
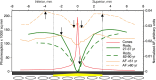
Methods: Retinal pigment epithelium-Bruch's membrane flat mounts were prepared from 20 donor eyes (10 ≤ 51 and 10 > 80 years; postmortem: ≤4.2 hours; no retinal pathologies), preserving foveal position. Phalloidin-binding RPE cytoskeleton and LF-AF (488-nm excitation) were imaged at up to 90 predefined positions. Maps were assembled from 83,330 cells in 1470 locations. From Voronoi regions representing each cell, the number of neighbors, cell area, and total AF intensity normalized to an AF standard was determined.
Results: Highly variable between individuals, RPE-AF increases significantly with age. A perifoveal ring of high AF mirrors rod photoreceptor topography and fundus-AF. Retinal pigment epithelium cell density peaks at the fovea, independent of age, yet no net RPE cell loss is detectable. The RPE monolayer undergoes considerable lifelong re-modeling. The relationship of cell size and AF, a surrogate for LF concentration, is orderly and linear in both groups. Autofluorescence topography differs distinctly from the topography of age-related rod loss.
Conclusions: Digital maps of quantitative AF, cell density, and packing geometry provide metrics for cellular-resolution clinical imaging and model systems. The uncoupling of RPE LF content, cell number, and photoreceptor topography in aging challenges LF's role in AMD.
Keywords: autofluorescence; cytoskeleton; lipofuscin; photoreceptor; retinal pigment epithelium.
Copyright 2014 The Association for Research in Vision and Ophthalmology, Inc.
Figures

References
-
- Ferris FL, Fine SL, Hyman L. Age-related macular degeneration and blindness due to neovascular maculopathy. Arch Ophthalmol. 1984; 102: 1640–1642 - PubMed
-
- Vingerling JR, Dielemans I, Hofman A, et al. The prevalence of age-related macuolpathy in the Rotterdam study. Ophthalmology. 1995; 102: 205–210 - PubMed
-
- Friedman DS, O'colmain BJ, Munoz B, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004; 122: 564–572 - PubMed
-
- Williams RA, Brody BL, Thomas RG, Kaplan RM, Brown SI. The psychosocial impact of macular degeneration. Arch Ophthalmol. 1998; 116: 514–520 - PubMed
-
- Strauss O. The retinal pigment epithelium in visual function. Physiol Rev. 2005; 85: 845–881 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

