Polymorphisms in catechol-O-methyltransferase modify treatment effects of aspirin on risk of cardiovascular disease
- PMID: 25035343
- PMCID: PMC4148908
- DOI: 10.1161/ATVBAHA.114.303845
Polymorphisms in catechol-O-methyltransferase modify treatment effects of aspirin on risk of cardiovascular disease
Abstract
Objective: Catechol-O-methyltransferase (COMT), a key enzyme in catecholamine metabolism, is implicated in cardiovascular, sympathetic, and endocrine pathways. This study aimed to confirm preliminary association of COMT genetic variation with incident cardiovascular disease (CVD). It further aimed to evaluate whether aspirin, a commonly used CVD prevention agent, modified the potential association of COMT with incident CVD.
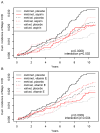
Approach and results: We examined COMT polymorphism rs4680 (MAF [minor allele frequency], 0.47), encoding a nonsynonymous methionine-to-valine substitution, in the Women's Genome Health Study (WGHS), a large population-based cohort of women with randomized allocation to aspirin or vitamin E when compared with placebo and 10-year follow-up. Rs4680 effects were confirmed with COMT polymorphism rs4818 and also examined in Coronary ARtery DIsease Genome-wide Replication and Meta-analysis/The Coronary Artery Disease Genetics Consortium, consortia for genome-wide association studies of coronary artery disease. Among WGHS women allocated to placebo (135 events/n=5811), the rs4680 valine allele was protective against incident CVD relative to the methionine (hazard ratio [HR; 95% confidence interval {CI}], 0.66 [0.51-0.84]; P=0.0007); an association also observed in Coronary ARtery DIsease Genome-wide Replication and Meta-analysis and The Coronary Artery Disease Genetics Consortium (combined P=2.4×10(-5)). In the WGHS, the rs4680 association was abolished by randomized allocation to aspirin, such that valine/valine women experienced higher CVD rates with aspirin allocation when compared with placebo (HR [95% CI], 1.85 [1.05-3.25]; P=0.033), whereas methionine/methionine women experienced lower rates (HR [95% CI], 0.60 [0.39-0.93]; P=0.023). Allocation to vitamin E also conferred higher but nonsignificant CVD rates on valine/valine (HR [95% CI], 1.50 [0.83-2.70]; P=0.180) when compared with significantly lower rates on methionine/methionine (HR [95% CI], 0.53 [0.34-0.84]; P=0.006) women. Rs4818 results were similar.
Conclusions: Common COMT polymorphisms were associated with incident CVD, and this association was modified by randomized allocation to aspirin or vitamin E. Replication of these findings is required.
Keywords: aspirin; catecholamines; vitamin E.
© 2014 American Heart Association, Inc.
Figures

References
-
- Voutilainen S, Tuomainen TP, Korhonen M, Mursu J, Virtanen JK, Happonen P, Alfthan G, Erlund I, North KE, Mosher MJ, Kauhanen J, Tiihonen J, Kaplan GA, Salonen JT. Functional comt val158met polymorphism, risk of acute coronary events and serum homocysteine: The kuopio ischaemic heart disease risk factor study. PLoS One. 2007;2:e181. - PMC - PubMed
-
- Zhu BT. On the mechanism of homocysteine pathophysiology and pathogenesis: A unifying hypothesis. Histol Histopathol. 2002;17:1283–1291. - PubMed
-
- Kanasaki K, Palmsten K, Sugimoto H, Ahmad S, Hamano Y, Xie L, Parry S, Augustin HG, Gattone VH, Folkman J, Strauss JF, Kalluri R. Deficiency in catechol-o-methyltransferase and 2-methoxyoestradiol is associated with pre-eclampsia. Nature. 2008;453:1117–1121. - PubMed
-
- Miyaki K, Htun NC, Song Y, Ikeda S, Muramatsu M, Shimbo T. The combined impact of 12 common variants on hypertension in japanese men, considering gwas results. J Hum Hypertens. 2012;26:430–436. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01AT004662/AT/NCCIH NIH HHS/United States
- K24AT004095/AT/NCCIH NIH HHS/United States
- R01 HL043851/HL/NHLBI NIH HHS/United States
- K24 AT004095/AT/NCCIH NIH HHS/United States
- F32 AT000051/AT/NCCIH NIH HHS/United States
- R01 CA047988/CA/NCI NIH HHS/United States
- HL080467/HL/NHLBI NIH HHS/United States
- UL1 RR025758/RR/NCRR NIH HHS/United States
- UL1 RR 025758/RR/NCRR NIH HHS/United States
- 3R01AT004662-02S1/AT/NCCIH NIH HHS/United States
- R21AT002860/AT/NCCIH NIH HHS/United States
- T32 AT000051/AT/NCCIH NIH HHS/United States
- CA047988/CA/NCI NIH HHS/United States
- R37 HL061795/HL/NHLBI NIH HHS/United States
- R21 AT002860/AT/NCCIH NIH HHS/United States
- R01 HL080467/HL/NHLBI NIH HHS/United States
- T32AT000051/AT/NCCIH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

